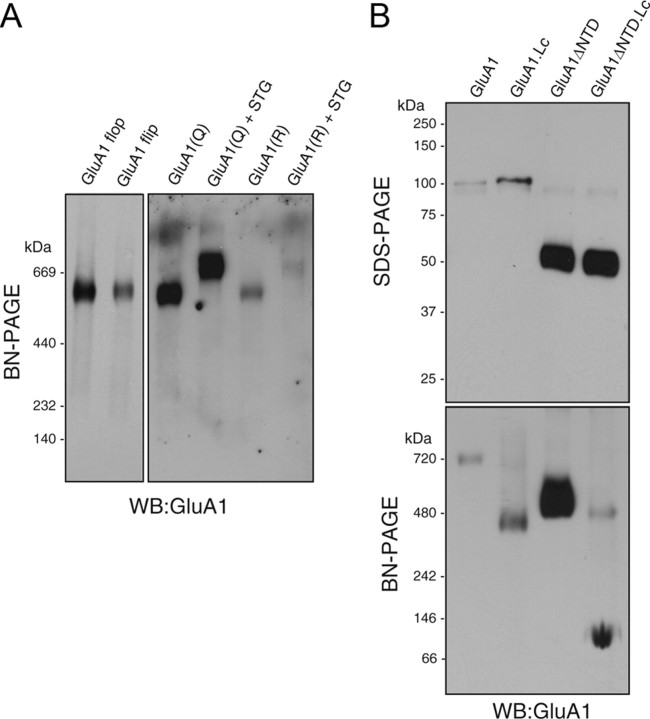
Figure 3.
The Lurcher site of GluA1, but not the Q/R editing site or the flip/flop splicing isoforms, was involved in the second dimerization of AMPA receptor dimers. Oocytes were injected with the cRNAs indicated. A, Both GluA1 flip and flop splicing isoforms (5 ng of cRNA each) elicited single bands at around 669 kDa on BN-PAGE (4–10%). In addition, both GluA1 and the Q/R edited forms (5 ng of cRNA each) yielded single bands at around 669 kDa on BN-PAGE. Coinjection with stargazin (STG; 0.4 ng of cRNA) led to an increase in the molecular weight to a similar extent. B, GluA1 and GluA1ΔNTD carrying a mutation (A636T) corresponding to the Lurcher mutation in the Δ2 orphan receptor (GluA1 Lc or GluA1ΔNTD Lc, 5 ng of cRNA each) were observed as single bands at around 100 and 50 kDa, respectively, on 10% SDS-PAGE, which was similar to what was observed for the wild type. Although GluA1 Lc and GluA1ΔNTD Lc assembled as a tetramer, the majority of GluA1 Lc and GluA1ΔNTD Lc were detected on BN-PAGE (3–15%) as a dimer and a monomer, respectively. WB, Western blotting.