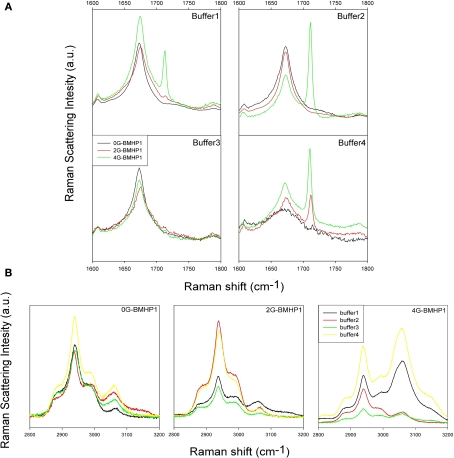
Figure 4.
Raman Spectra of tested self-assembling peptides. (A) Raman Spectra (1600–1800 cm−1 region) of the tested self-assembling peptides. Each spectrum shows the peptide Amide I region in all buffered solutions. In buffer 1, buffer 2 and buffer 3, each peptide shows a similar conformation of Amide I peak, suggesting a similar β-sheet conformation of the peptide centered in 1675 cm−1. In buffer 4 the pH shift alters the self-assembled structure of 0G-BMHP1 thus affecting the shape of its Amide I peak and suggesting a wider unordered component. (B) Raman Spectra of 2500–3100 cm−1 region of the tested self-assembling peptides (region of νCH2) exposed to different buffers. The change in the relative intensity between the peak at ∼2940 cm−1 (νCH2) and the peak at ∼3061 cm−1 νCH2 of Phe can be appreciated in case of peptide 4G BMHP1.