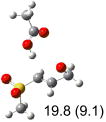
Table 1.
Calculated transition states, reaction barriers and reaction enthalpies in methanol solution for different conformers of the model inhibitorsa,b
| thiirane | oxirane | |
|---|---|---|
| (a) | 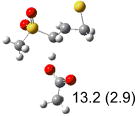 |
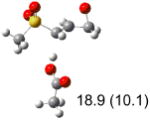 |
| (b) | 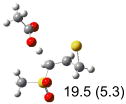 |
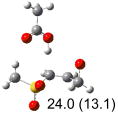 |
| (c) | 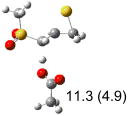 |
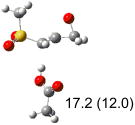 |
| (d) | 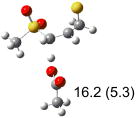 |
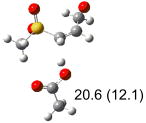 |
| (e) | 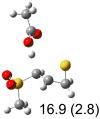 |
 |
| (f) | 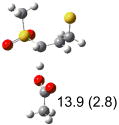 |
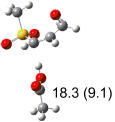 |
IEF-PCM/B3LYP/6-311+G(d,p) energies with optimized geometries and zero point energies obtained with the 6-31+G(d) basis set. The reaction enthalpies are listed in the parenthesis. The color scheme is the same as in Figure 1.
Using the most stable conformer for the reactant complex of the R and S enantiomers of 1 for reactants, and infinitely separated acetic acid and ring opened structures for products.
