Figure 5.
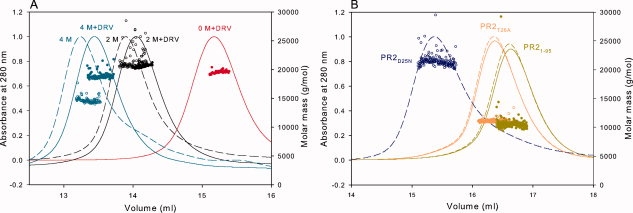
Analytical size-exclusion chromatography with inline multiangle light scattering and refractive index measurements (SMR) of PR2 and its mutants in 50 mM sodium acetate, pH 5, 50 mM NaCl. Proteins were folded as described in “Materials and Methods.” Based on the width at half peak height, the concentrations of proteins sampled for light scattering, refractive index, and UV measurements are expected to be roughly 6 μM. Molecular masses (circles) were calculated using the Astra software provided by Wyatt Technology Inc. (A) The final concentrations of urea in the sample and column buffer are 0M (red), 2M (black), and 4M (cyan). Dashed lines are in the absence of inhibitor; continuous lines are in the presence of a 1.1-fold excess of DRV added to the sample prior to chromatography. (B) Fractionation profiles and molecular mass values of PR2D25N (blue), PR2T26A (orange), and PR21-95 (dark yellow). PR2T26A and PR21-95 fractionated after the addition of 1.1-fold excess DRV, shown in continuous lines, exhibit monomer masses (see Table I) similar to proteins fractionated without DRV (dashed lines). Molar masses are shown as open and closed circles for the samples in the absence and presence of DRV, respectively. Several features of the chromatograms are worth noting: (1) In the absence of urea, dimeric PR2 and PR2D25N both elute in a retention volume slightly greater than 15 mL, whereas monomeric PR2T26A and PR21-95 elute significantly later as expected. Interestingly, the deletion mutant PR21-95, with only a ∼4% lower monomer mass, elutes slightly later than PR2T26A. (2) Urea in the column eluant causes the dimers to be less strongly retained and shifts the elution to significantly smaller volumes, possibly by altering interactions of the protein with the column matrix. (3) Binding of the inhibitor DRV in the presence (A) or absence of urea shifts the elution to slightly greater volumes relative to the unliganded protein but has no effect on the elution of monomers PR2T26A and PR21-95, with which it presumably does not interact.
