Abstract
Ecosystems can alternate suddenly between contrasting persistent states due to internal processes or external drivers. It is important to understand the mechanisms by which these shifts occur, especially in exploited ecosystems. There have been several abrupt marine ecosystem shifts attributed either to fishing, recent climate change or a combination of these two drivers. We show that temperature has been an important driver of the trophodynamics of the North Sea, a heavily fished marine ecosystem, for nearly 50 years and that a recent pronounced change in temperature established a new ecosystem dynamic regime through a series of internal mechanisms. Using an end-to-end ecosystem approach that included primary producers, primary, secondary and tertiary consumers, and detritivores, we found that temperature modified the relationships among species through nonlinearities in the ecosystem involving ecological thresholds and trophic amplifications. Trophic amplification provides an alternative mechanism to positive feedback to drive an ecosystem towards a new dynamic regime, which in this case favours jellyfish in the plankton and decapods and detritivores in the benthos. Although overfishing is often held responsible for marine ecosystem degeneration, temperature can clearly bring about similar effects. Our results are relevant to ecosystem-based fisheries management (EBFM), seen as the way forward to manage exploited marine ecosystems.
Keywords: benthic, cod, plankton, regime shift, temperature, trophic cascade
1. Introduction
Ecosystems exist as dynamic regimes controlled by the interplay among species, the environment and how they interact with external forces such as climate (Scheffer & Carpenter 2003). Many studies have reported the rapid alteration of marine ecosystems throughout the world (Pauly et al. 1998; Oguz 2007; Österblom et al. 2007). Although human activities and especially fishing are often held responsible for these abrupt ecosystem shifts (Frank et al. 2005; Weijerman et al. 2005; deYoung et al. 2008; Jackson 2008; Casini et al. 2009), the oceanic biosphere is now also experiencing rapid global climatic change (Brander 2007). Recently, several studies have described pronounced and sustained responses of marine ecosystems to climate warming (IPCC 2007). Although it has been suggested that marine ecosystems should be resilient to exploitation when managed effectively (Hughes et al. 2005) the steady decline of most managed neritic fisheries seems to suggest otherwise (Frank et al. 2005). One possible explanation for why ecosystem resource management is problematic could be the difficulty of separating the synergistic effects of fishing from climate (Kirby et al. 2009). Indeed, the complexity of living systems has caused some to ask whether ecosystem-based fisheries management (EBFM) in marine ecosystems (Pikitch et al. 2004) is even achievable (Longhurst 2006).
The North Sea ecosystem provides 5 per cent of the global fish harvest and has been fished heavily with a particular effect on cod (Gadus morhua L.) (Heath 2005). We have previously shown that temperature is more important than wind intensity and direction, salinity, nutrients and oxygen in determining the North Atlantic and North Sea ecosystem dynamic regime (Beaugrand et al. 2008). During the 1980s, the North Sea experienced a change in hydro-climatic forcing that caused a rapid, temperature-driven ecosystem shift (Beaugrand & Ibañez 2004). This change in sea surface temperature (SST) altered the plankton and affected the recruitment of cod negatively, at a time when their stocks were also experiencing overfishing (Beaugrand et al. 2003; Heath 2005). Changes in the North Sea plankton, following the ecosystem shift, include an increase in microalgae (Kirby et al. 2008), a change in the composition and abundance of the holozooplankton (Beaugrand et al. 2003), increases in the frequency of jellyfish (Kirby et al. 2009) and in the abundance of decapod and echinoderm larvae, and a decrease in bivalve larvae (Kirby et al. 2008).
Extensive biological datasets exist for the North Sea that make it uniquely possible to apply an end-to-end ecosystem approach (Travers et al. 2007) to investigate the effect of temperature on five trophic levels, primary producers (microalgae), primary, secondary and tertiary consumers (zooplankton, fish and jellyfish), and benthic detritivores (echinoderms and bivalves) (some of these taxa may occupy different trophic levels at different life history stages). These taxa include important commercial species, demersal cod and the benthic flatfish, plaice (Pleuronectes platessa L.) and sole (Solea solea L.), and incorporate predator–prey interactions that are known to structure pelagic and benthic communities (cod–decapods, Frank et al. 2005; decapods–flatfish, van der Veer & Bergman 1987; decapods–bivalves, Barkai & McQuaid 1988). The dataset also contains several taxa whose planktonic larvae are indicators of benthic–pelagic coupling (decapods, echinoderms and bivalves). From this dataset we derived 16 biological descriptors of the North Sea ecosystem that we used to analyse long-term changes for the period 1958 to 2005 by standardized principal component analysis (PCA). To understand the mechanisms underlying the relationship between temperature and ecosystem structure we applied causal modelling (Legendre & Legendre 1998); this is a statistical method that can be used to determine the type of control in an ecosystem (bottom-up or top-down; Cury et al. 2003). In this way, we probed much deeper than current proposed approaches (Cury et al. 2003). Our results show that temperature has been an important driver of North Sea trophodynamics for nearly 50 years and that a recent change in temperature has established a new ecosystem dynamic regime by modifying the strength and direction of some trophic interactions.
2. Material and methods
(a). Biological data
Plankton data were collected by the Continuous Plankton Recorder (CPR) survey. The CPR survey has operated in the North Sea on a routine monthly basis since 1946. Seawater enters the CPR through a front aperture and the plankton is retained on a moving band of silk gauze of mesh size 270 µm that is slowly wound into a tank of formalin. In the laboratory the silk gauze is cut into sections (a CPR sample), each representing the plankton from 3 m3 of water taken during 10 nautical miles (18 km) of tow at an average depth of 7 m. Up to 450 taxa are identified and enumerated. The methods of CPR sampling and analysis have remained consistent throughout the time series (Batten et al. 2003). The plankton data we used comprise the abundance of the holozooplanktonic copepods Calanus finmarchicus and Pseudocalanus spp., a measure of the total holozooplankton, and the abundance of the merozooplanktonic larvae of decapods, echinoderms and bivalves. The dataset also includes the frequency of jellyfish material estimated by nematocyst frequency. An index of holozooplankton composition was also created using a standardized PCA. An annual mean was first calculated for all holozooplanktonic species or taxonomic groups. Then, species or taxonomic groups with an annual relative abundance >0.001 and a presence >30 per cent for all years in the period 1958 to 2005 were selected. This procedure allowed the selection of 35 holozooplankton species or taxonomic groups. Abundance data in the matrix (48 years × 35 species or taxonomic group) were transformed using the function log10(x + 1). A PCA was then performed on the correlation matrix (35 × 35 species) to identify the main pattern of long-term changes in holozooplankton community structure (examination of principal components). An estimate of the amount of chlorophyll in each CPR sample was derived from the phytoplankton colour index that measures the greenness of the silk due to both trapped cells and fragile phytoplankton taxa that burst upon impact. The phytoplankton colour index correlates well with both fluorometer and satellite measures of chlorophyll (Raitsos et al. 2005). Data on North Sea demersal fish (cod, plaice and sole spawning stock biomass (SSB) and recruits) were obtained from http://www.ices.dk and are derived from a virtual population analysis based on fisheries data. We restricted our analysis to commercially fished demersial species, because, with the exception of the herring (Clupea harengus), similar long-term datasets for small pelagic species such as sandeel (Ammodytes marinus) and sprat (Sprattus sprattus) do not exist and to shorten our time series to accommodate these species would have limited the power of causal modelling. From our plankton and fish dataset we derived 16 biological descriptors of the North Sea ecosystem (figure 1).
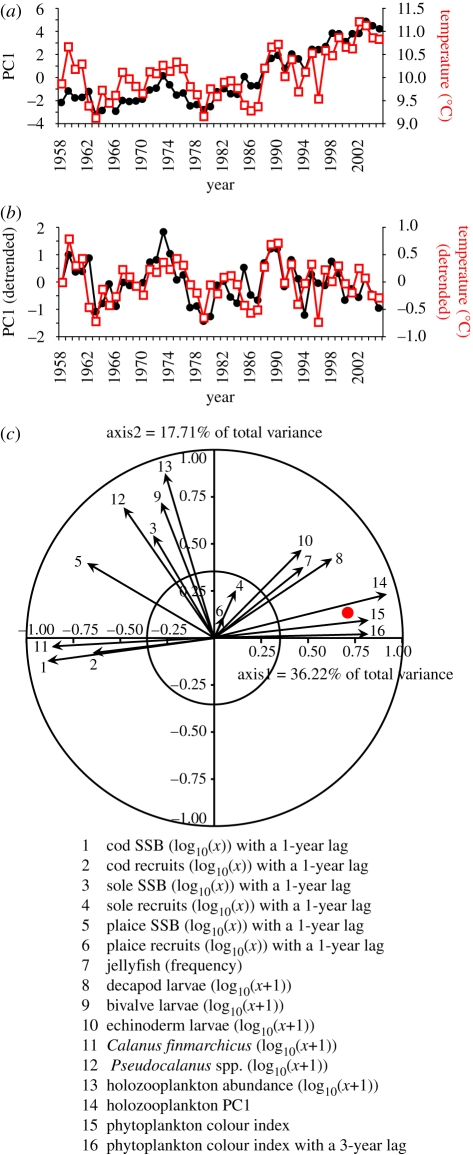
Figure 1.
Principal component analysis on long-term changes in the North Sea ecosystem. (a) Relationship between the first principal component (36.22% of the total variance) in black dots and annual sea surface temperature (SST) in red squares. (b) Relationship between the detrended first principal component and detrended annual SST. (c) Normalized eigenvectors 1 and 2 (53.93% of the total variance); both circle of correlation (outer circle) and circle of equilibrium (inner circle) descriptor contribution (c = 0.353) are displayed. Variables inside the latter circle have a non-significant contribution. Annual SST (red dot) is a supplementary variable and does not contribute to the principal components. A total of 16 biological variables were included in the analyses. A three-year lag was introduced into the phytoplankton colour index due to the positive correlation that exists between this variable and decapod larval abundance (Kirby et al. 2008).
(b). Physical data
Annually averaged sea surface temperature data for the North Sea were calculated from the COADS 1-degree enhanced dataset provided by the comprehensive NOAA-CIRES Climate Diagnostics Center Database (Boulder, Colorado, USA) (Woodruff et al. 1988).
(c). Statistical analysis
All plankton abundance data were first transformed using the function, log10(x + 1). Fish data were transformed using the function, log10(x). Long-term changes in the ecosystem state were first studied by means of principal components. Relationships between indices of ecosystem change (e.g. principal components) and SST were then investigated by correlation analysis. Correlation analyses were performed on both original and detrended data to examine the relationships between temperature and ecosystem change more closely (figure 1a,b). A one-year lag was introduced when the correlations were calculated between fish data (SSB and recruits) and plankton or SST. A three-year lag was introduced between decapod larvae and the phytoplankton colour index (Kirby et al. 2008). The time series were detrended by means of singular spectrum analysis (SSA) (Vautard & Ghil 2002). This method uses a principal component analysis performed on an autocovariance matrix (also called a Toeplitz matrix) to divide a time series into a succession of signals of decreasing variance (Beaugrand & Reid 2003). The method of SSA is also known both as eigenvector filtering (EVF) and PCA of processes. SSA decomposes a signal into different components (long-term trends, cycles or pseudocycles and year-to-year variability) and it has been applied usefully in marine ecology before (Ibanez & Etienne 1992; Molinero et al. 2005). Detrended time series were calculated by subtracting from each original time series its respective long-term trend identified by the first principal component of the SSA. Probabilities were calculated with consideration to a modified temporal Box–Jenkins autocorrelation function (Chatfield 1996) with adjusted degrees of freedom (Chelton 1984). Relationships between each biological descriptor and PC1 were assessed by examining the eigenvectors (figure 1c).
The linear coefficients of first-order partial correlation were then calculated to determine how a climatic signal propagates into the food web, and the results presented synthetically on a global diagram (figure 2a). The partial correlation coefficients allow the relationship between two variables to be measured after removing the effect of a third variable while keeping its mean constant. The partial correlation coefficient between variables 1 and 2, removing the linear effect of variable 3 (noted r12.3), was calculated using the equation provided by Legendre and Legendre (1998).
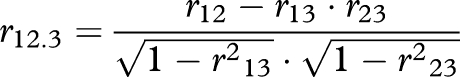 |
where r12, r13 and r23 are simple linear coefficients of correlation between 1 and 2, 1 and 3, and 2 and 3, respectively. A simple way of studying statistical causal relationships may be carried out on three variables by looking at both ordinary and partial correlation coefficients (Legendre & Legendre 1998). Using four reference models (see figure S1 in the electronic supplementary material), we determined causal relationships between variables by applying the technique to each possible triplet of variables. By calculating both the ordinary correlation and the first-order partial correlation coefficients, causal modelling allows for the detection of spurious correlations, which is an important feature of the method. For example, in electronic supplementary material figure S1, model 2, the ordinary correlation between variables 2 and 3 can be found to be significant, because variable 1 influences both variable 2 and variable 3. By calculating the first-order partial coefficient of correlation (r23.1) this effect can be detected. Because causal modelling adds other criteria to validate the model, such as the interaction factor r12 × r13 = r23, spurious correlations are minimized.
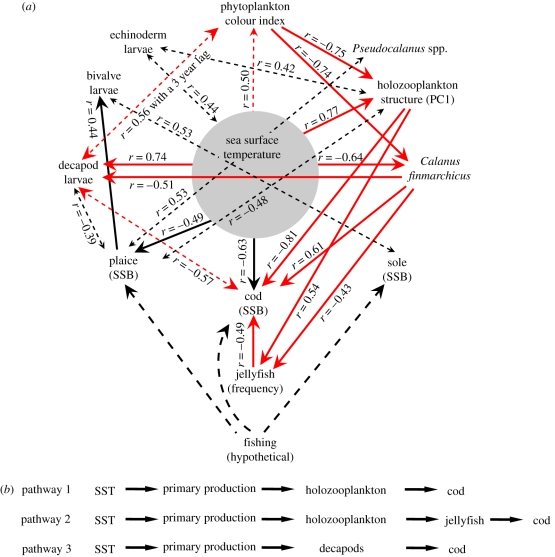
Figure 2.
Statistical causal modelling to determine the pathways by which temperature propagates through the North Sea food web. (a) Causal model based on the examination of both ordinary and first-order partial coefficients of correlation between 12 variables. Only significant ordinary coefficients of correlation (p < 0.05) are indicated on the diagram. Unidirectional solid arrows indicate a statistical causal link between variables with an indication of direction. Bidirectional dashed arrows indicate a correlation between variables without direction. Red arrows indicate pathways contributing to the trophic amplification of temperature effects with respect to cod. Note that the red arrow between sea surface temperature (SST) and phytoplankton is shown dashed as the analysis did not indicate direction in this case. However, we consider it unlikely that phytoplankton influences SST over the timescale of our study. Black, unidirectional, dashed arrows suggest the top-down effects of fishing. (b) Three indirect pathways leading to the trophic amplification of temperature on cod.
Finally, sliding correlation analysis was applied to assess the temporal stability of the relationships among variables for a time window of 20 years. This approach calculates correlations for moving time periods with an increment of one year. As the technique is sensitive to the chosen time period, windows were examined ranging between 10 and 25 years; these all gave similar conclusions. First, the technique was applied between the first principal component and the 16 biological variables (figure 3). Second, the technique was used on selected pairs of variables (figure 4).
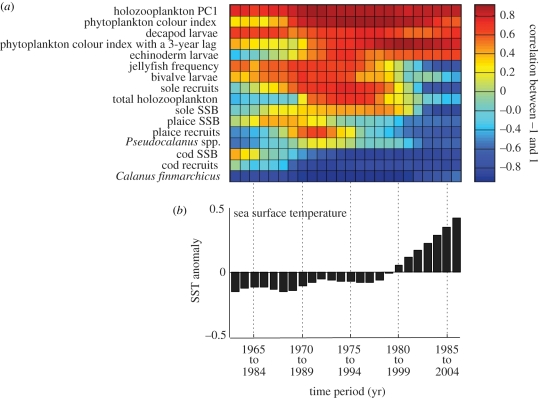
Figure 3.
Examination of the effect of temperature on the relationships among species and trophic levels for the period 1958 to 2005. (a) Sliding correlation analysis between the first principal component and the 16 biological descriptors of the North Sea ecosystem using a time window of 20 years. (b) Sliding average of annual sea surface temperature (SST) using a time window of 20 years. Each vertical bar represents a 20 year period; for example, the first three vertical bars represent the overlapping periods 1963 to 1982, 1964 to 1983, and 1965 to 1984, respectively. Consequently, in the axis labels the upper and lower years denote the beginning and the end of the time period, respectively.
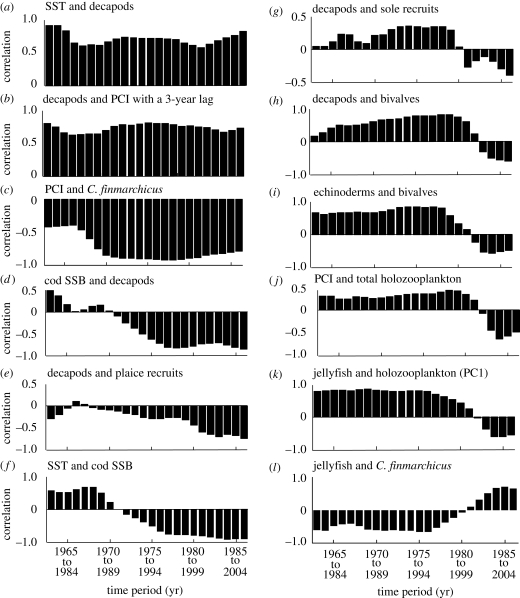
Figure 4.
Sliding correlation analysis between pairs of variables for the period 1958 to 2005. (a) sea surface temperature (SST) and decapods. (b) Decapods and phytoplankton colour index (PCI) with a three-year lag. (c) PCI and C. finmarchicus. (d) Cod SSB and decapods. (e) Decapods and plaice recruits. (f) SST and cod SSB. (g) Decapods and sole recruits. (h) Decapods and bivalves. (i) Echinoderms and bivalves. (j) PCI and total holozooplankton. (k) Jellyfish and holozooplankton (PC1). (l) Jellyfish and C. finmarchicus. Each vertical bar represents a 20 year period, for example, the first three vertical bars represent the overlapping periods 1963 to 1982, 1964 to 1983, and 1965 to 1984, respectively. Consequently, in the axis labels, the upper and lower years denote the beginning and the end of the time period, respectively.
3. Results
The PCA performed on the table of 16 biological indicators of the North Sea ecosystem suggested a strong influence of temperature on the ecosystem dynamic regime (figure 1a) (r = 0.74, p = 0.002, probability corrected for temporal autocorrelation) as indicated by the first principal component (36.22% of the total variance). This positive link is still visible when both time series are detrended (r = 0.59, p = 0.0001, probability corrected for autocorrelation; figure 1b). Examination of the eigenvectors shows that the influence of temperature pervades the whole ecosystem (figure 1c).
Causal modelling revealed that temperature acts through several direct and indirect pathways (figure 2a) to influence the trophodynamics of the North Sea ecosystem. A total of five direct (statistical) causal links between SST and biological parameters were identified (holozooplankton structure, abundance of Calanus finmarchicus, cod SSB, plaice SSB and decapod larvae) and two correlations between SST and the phytoplankton colour index and echinoderm larvae. Taking cod as an example of the complexity of the system, we see that temperature acts both directly (negative effect) and indirectly via the food web (negative effects). This can be summarized in the following way:
 |
where n is the number of considered species, Etotal the total influence of temperature on the target species (e.g. cod), Etemperature the direct influence of temperature on the target species and Ei the influence of temperature through the food web.
Indirect pathways include (i) the effects of temperature on the zooplankton prey of larval cod (figure 2b, pathway 1), (ii) through decapods (figure 2b, pathway 3), which may operate through decapod predation on holozooplankton (Frank et al. 2005), and (iii) through predation by jellyfish (figure 2b, pathway 2). For example (see figure S1, model 4 in the electronic supplementary material), in the case of SST (variable 1), the preferred prey of cod, C. finmarchicus (variable 2) and cod (variable 3), both the ordinary coefficients of correlation are significant (r12 = −0.64 (p12 < 0.0001); r13 = −0.63 (p13 < 0.0001); r23 = 0.61 (p23 < 0.0001)) and the first-order partial coefficients of correlation are significant (r12.3 = −0.50 (p12.3 = 0.0007); r13.2 = −0.36 (p13.2 = 0.02); r23.1 = −0.31 (p23.1 = 0.04)). Therefore, the negative influence of temperature on cod is direct and also indirect through its negative influence on C. finmarchicus, which is positively related to cod.
When sliding correlation analysis was applied between the first principal component (figure 1a) and each of the 16 biological variables with a moving window of 20 years, the relationship between the first principal component and some variables was found to be constant (figure 3a), for example, C. finmarchicus and cod recruits (both negatively correlated with the first principal component). Other biological variables in the North Sea ecosystem appear to either develop through time (e.g. echinoderm larvae), or show a change in the sign of their relationship after the beginning of the 1980s (e.g. jellyfish frequency) (figure 3a). The changes in these variables coincide with a rapid and sustained increase in temperature (figure 3b).
A pairwise analysis of the relationships among biological variables reveals three types of interaction (figure 4). The first is a constant interaction over time (i.e. at least the time period considered); this is shown by the relationships between SST and decapod larvae (figure 4a), decapod larvae and the phytoplankton colour index with a three-year-lag (figure 4b), and C. finmarchicus and phytoplankton colour (figure 4c), for example. The second type of interaction is where a significant relationship develops such as the negative relationship between cod SSB and decapod larvae (figure 4d). A strengthening negative relationship also develops between plaice and their decapod predators (figure 4e). The third type of interaction is where the sign of the relationship reverses (figure 4f–l).
4. Discussion
Temperature, through its effect on physiology, can modulate species distributions, interactions and trophodynamics (Cury et al. 2008). Unfortunately, there are few ecosystems where sufficient data exist to examine the effect of temperature across several trophic levels and long timescales. The North Sea dataset used in this study is unique in this respect. Our results show that temperature has been an important mechanism (figure 1) driving the trophodynamics (figure 2) of this heavily fished marine ecosystem for nearly 50 years. A recent pronounced change in temperature (figure 3) appears to have established a new ecosystem dynamic regime through a series of internal mechanisms (figure 4). Our analysis of the North Sea ecosystem indicates that the influence of temperature translates through the food web to the ecosystem level. It is, therefore, unsurprising that climate change is held responsible for several recent abrupt ecosystem shifts, especially in the marine environment where the plankton food web is especially sensitive to hydroclimatic change (Beaugrand et al. 2003).
Positive correlations between ecosystem dynamic regimes and the hydroclimatic environment have been reported before (Weijerman et al. 2005), but until now it had remained unclear how temperature might propagate through the ecosystem to affect energy allocation, predator–prey interactions and benthic–pelagic coupling (Cury et al. 2008). By using causal modelling we have revealed how the food web can propagate the effect of temperature through a number of indirect pathways. We call this intensification of the effect of temperature through indirect pathways a trophic amplification. Trophic amplification represents an alternative to positive feedback as a mechanism by which abrupt ecosystem shifts occur (Scheffer & Carpenter 2003). It is also distinct to true positive feedback, because, in our case, we have not considered any possible influence of plankton on climate (Lovelock 1979; Charlson et al. 1987). At the level of the ecosystem, trophic amplification intensifies the effect of climate warming, potentially leading to a new attractor (ecosystem dynamic regime) (Scheffer & Carpenter 2003). This could explain the often nonlinear and sometimes unpredictable response of ecosystems to climate variability (Muradian 2001; Taylor et al. 2002; Hsieh et al. 2005). As a top predator, cod seem particularly vulnerable to trophic amplification of the effects of temperature (figure 2b). Even though certain conditions might seem to favour cod, such as the increase in decapod prey in the benthos (Kirby et al. 2008, 2009), the amplified effect of temperature, especially on the larval stage (a critical phase in the life cycle of fish; Cushing 1990), is overwhelming.
Rapid shifts between alternative attractors occur in lakes when temperature affects the development of the clear-water phase to modify the plankton food web (Scheffer & Carpenter 2003). A sliding correlation analysis revealed that there was a differential response among biological variables to a pronounced change in temperature; some pairs of variables were found to be constant, whereas others developed or showed a change in the sign of their relationship (figure 3). This differential response is a clear manifestation of the effects of the North Sea abrupt ecosystem shift (Weijerman et al. 2005) on the trophodynamics of the system. This analysis also revealed that the main component of the food web, with respect to North Sea cod (the copepod, C. finmarchicus; Beaugrand et al. 2003), is stable through time. Consequently, a single causal modelling (figure 2a) for the whole time period was sufficient to understand the effects of the trophic amplification of temperature in the food chains involving cod.
Although we did not include the effect of fishing in our analyses (we found that the effect of cod fishing mortality on the first principal component was not significant, r = 0.55, p = 0.197), the removal of top predators can also alter ecosystems by triggering trophic cascades (Pace et al. 1999), and in pelagic marine ecosystems, overfishing is considered a likely cause (Daan et al. 2005; Frank et al. 2005; Scheffer et al. 2005; Casini et al. 2008). Our time series begins around the time of the disappearance of the high trophic level Atlantic bluefin tuna (Thunnus thynnus) from the North Sea in the early 1960s; prior to this, from 1900 to 1950, there was a commercial tuna fishery in the region (MacKenzie & Myers 2007). Consequently, the trophic hierarchy of the North Sea ecosystem was altered near the start of our time series. Cod have also been fished heavily in the North Sea (Heath 2005), and where this has occurred elsewhere, it has been held responsible for alterations in other trophic components of the ecosystem (Frank et al. 2005), especially when coupled with unfavourable recruitment conditions for cod (Casini et al. 2009). Although temperature appears an important driver of both plankton and cod SSB in the North Sea, overfishing may nevertheless have modulated the ecosystem dynamic regime through the interaction between bottom-up and top-down effects in the plankton (Stige et al. 2009). In particular, fishing may have enabled an increase in gelatinous plankton in the North Sea, as occurred in the Black Sea (Daskalov et al. 2007), with consequent effects on both plankton and fish recruitment (figure 2a; Kirby et al. 2009).
The pairwise analysis of the relationships among biological variables showed three types of interaction in the ecosystem (figure 4). The first, such as the relationship between decapod larvae and SST (figure 4a), and the relationship between decapod larvae and phytoplankton colour index with a three-year lag (figure 4b) was constant through time and has been seen before (Kirby et al. 2008). These relationships are likely to reflect the positive influence of temperature and food on decapod reproductive output and larval survival (Kirby et al. 2008). Although the relationship between larval abundance and the size of benthic populations has not been generally established for invertebrate macrofauna, larval surveys are a long established means of estimating the spawning stocks of decapods (Briggs et al. 2002). The increase in decapod larval abundance in CPR samples reflects changes in larval numbers of Polybiinae (swimming crab larvae), Upogebia deltaura, Callianassa subterranea and Cancer pagurus (Rees 1955; Lindley et al. 1993), predominantly. Fisheries data show that landings of the predatory decapods Pandalus and Nephrops, (ICES 2006) and Cancer (Heath 2005) have increased markedly in the North Sea, and Rees et al. (2007) also noted increases in U. deltaura and C. subterranea in certain regions.
The second interaction we observed is where a significant relationship develops through time, such as the negative relationship between cod SSB and decapod larvae (figure 4d), which may indicate the opposite influences of temperature on both cod recruitment (Beaugrand et al. 2003) and decapod larval abundance (Kirby et al. 2008), and reduced predation by adult cod on decapods (Frank et al. 2005). A strengthening negative relationship also develops between both plaice and their decapod predators (figure 4e), which may reflect increased decapod numbers and predation on plaice recruits (van der Veer & Bergman 1987); this may also explain the change in the relationship between decapods and sole recruits (figure 4g, see below). Interestingly, this suggests that different fisheries may be linked through changes in the food web. However, it is the third type of interaction, where the sign of the relationship reverses around an ecological threshold (figure 4f–l), that is perhaps the most interesting, as it reveals nonlinearity in the system. For example, the relationship between cod SSB and temperature is positive during the cold-water phase (1962–1982) of the North Sea and becomes negative during the warm-water phase (1989 onwards; Beaugrand 2004; figure 4f). The effect of temperature on cod may reflect the thermal tolerance of the preferred prey of larval cod, for example, C. finmarchicus (figure 2; Beaugrand et al. 2003). These results re-emphasize how internal processes may amplify a small change in an external driver, such as a 1°C temperature change (Beaugrand 2004), to overcome an ecological threshold and lead to a new attractor.
The effect of temperature may have also influenced a number of internal processes such as predator–prey interactions. Examples of changes in interspecific relationships include the change in the sign of the relationships between decapods and sole (figure 4g) and between decapods and bivalves (figure 4h), which may reflect increased predation by decapods in the benthos (van der Veer & Bergman 1987; Barkai & McQuaid 1988; Kirby et al. 2008). Like decapods, the larval production of benthic echinoderms is also affected positively by North Sea temperature (Kirby et al. 2008) and so it is unsurprising that the trend between echinoderms and bivalves (figure 4i) is similar to that between decapods and bivalves (figure 4h). Numbers of the dominant echinoderm in CPR samples, the psammivorous detritivore, Echinocardium cordatum (Kirby et al. 2007), have also increased in the benthos (Rees et al. 2007). Changes in the relationships between microalgae and holozooplankton (figure 4j), and between jellyfish and holozooplankton PC1 (figure 4k) could reflect trophic interactions similar to those suggested to have occurred in other ecosystems (Frank et al. 2005; Daskalov et al. 2007). The opposite relationships between jellyfish and holozooplankton PC1, and jellyfish and C. finmarchicus (figures 2a and 4k,l) can be explained by the strong negative relationship that exists between these two zooplankton components. Holozooplankton PC1 represents smaller zooplankton than C. finmarchicus, which show opposite patterns of long-term change due to the influence of temperature (figure 1c). Taken together, these alterations in the strength and sign of the relationships among taxa are a clear manifestation of nonlinear dynamics in an ecosystem (Muradian 2001; Scheffer & Carpenter 2003).
In the North Sea the new dynamic regime favours jellyfish in the plankton and decapods and detritivores (echinoderms) in the benthos (Kirby et al. 2008, 2009). Although fishing down marine food webs has previously been held responsible for their degeneration (Pauly et al. 1998), temperature can clearly bring about the same effect. So far, the North Sea has only experienced a 1°C change in SST and this is expected to double by the end of the century (Scenario A2, IPCC 2007). The societal demand for managing marine resources compels us to increase our efforts to understand the complexities of the ecosystem. Our results show that bottom-up control is an important influence on a marine ecosystem and, in the case of the North Sea, on the important commercial species, cod (direct and indirect effects), plaice and sole (indirect effects), and so climate change should be considered along with the effect of fishing in EBFM. The nonlinear dynamics and the complexity of possible trajectories among components of the ecosystem simply make the application of EBFM more challenging (Longhurst 2006; deYoung et al. 2008).
Acknowledgements
We are grateful to all past and present staff of the Sir Alister Hardy Foundation for Ocean Science and the Masters and crews of the ships that tow CPRs on a voluntary basis. R.R.K. is a Royal Society University Research Fellow. We thank two anonymous referees for their helpful comments on our manuscript.
References
- Barkai A., McQuaid C.1988Predator–prey role reversal in a marine benthic ecosystem. Science 242, 62–64 (doi:10.1126/science.242.4875.62) [DOI] [PubMed] [Google Scholar]
- Batten S. D., et al. 2003CPR sampling: the technical background, materials and methods, consistency and comparability. Prog. Oceanogr. 58, 193–215 (doi:10.1016/j.pocean.2003.08.004) [Google Scholar]
- Beaugrand G.2004The North Sea regime shift: evidence, causes, mechanisms and consequences. Prog. Oceanogr. 60, 245–262 [Google Scholar]
- Beaugrand G., Brander K. M., Lindley J. A., Souissi S., Reid P. C.2003Plankton effect on cod recruitment in the North Sea. Nature 426, 661–664 (doi:10.1038/nature02164) [DOI] [PubMed] [Google Scholar]
- Beaugrand G., Edwards M., Brander K., Luczak C., Ibañez F.2008Causes and projections of abrupt climate-driven ecosystem shifts in the North Atlantic. Ecol. Lett. 11, 1157–1168 [DOI] [PubMed] [Google Scholar]
- Beaugrand G., Ibañez F.2004Monitoring marine plankton ecosystems (2): long-term changes in North Sea calanoid copepods in relation to hydro-climatic variability. Mar. Ecol. Prog. Ser. 284, 35–47 (doi:10.3354/meps284035) [Google Scholar]
- Beaugrand G., Reid P. C.2003Long-term changes in phytoplankton, zooplankton and salmon linked to climate. Global Change Biol. 9, 801–817 (doi:10.1046/j.1365-2486.2003.00632.x) [Google Scholar]
- Brander K. M.2007Global fish production and climate change. Proc. Natl Acad. Sci. USA 104, 19 709–19 714 (doi:10.1073/pnas.0702059104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs R. P., et al. 2002The application of fecundity estimates to determine the spawning stock biomass of Irish Sea Nephrops norvegicus (L.) using the annual larval production method. ICES J. Mar. Sci. 59, 109–119 (doi:10.1006/jmsc.2001.1144) [Google Scholar]
- Casini M., et al. 2008Multi-level trophic cascades in a heavily exploited open marine ecosystem. Proc. R. Soc. B 275, 1793–1801 (doi:10.1098/rspb.2007.1752) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casini M., et al. 2009Trophic cascades promote threshold-like shifts in pelagic marine ecosystems. Proc. Natl Acad. Sci. USA 106, 197–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson R. J., Lovelock J. E., Andreae M. O., Warren S. G.1987Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate. Nature 326, 655–661 (doi:10.1038/326655a0) [Google Scholar]
- Chatfield C.1996The analysis of time series: an introduction London, UK: Chapman and Hall/CRC [Google Scholar]
- Chelton D. B.1984Commentary: short-term climatic variability in the northeast Pacific Ocean. In The influence of ocean conditions on the production of salmonids in the North Pacific (ed. Pearcy W.), pp. 87–99 Corvallis, OR: Oregon State University Press [Google Scholar]
- Cury P., Shannon L., Shin Y. J.2003The functioning of marine ecosytems: a fisheries perspective. In Responsible fisheries in the marine ecosystem (eds Sinclair M., Valdimarsson G.), pp. 103–123 Wallingford, UK: CABI Publishing [Google Scholar]
- Cury P. M., et al. 2008Ecosystem oceanography for global change in fisheries. Trends Ecol. Evol. 23, 338–346 (doi:10.1016/j.tree.2008.02.005) [DOI] [PubMed] [Google Scholar]
- Cushing D. H.1990. Plankton production and year-class strength in fish populations: an update of the match/mismatch hypothesis. Adv. Mar. Biol. 26, 249–292 (doi:10.1016/S0065-2881(08)60202-3) [Google Scholar]
- Daan N., Gislason H., Pope J. G., Rice J. C.2005Changes in the North Sea fish community: evidence of indirect effects of fishing? ICES J. Mar. Sci. 62, 177–188 (doi:10.1016/j.icesjms.2004.08.020) [Google Scholar]
- Daskalov G. M., Grishin A. N., Rodionov S., Mihneva V.2007Trophic cascades triggered by overfishing reveal possible mechanisms of ecosystem regime shifts. Proc. Natl Acad. Sci. USA 104, 10 518–10 523 (doi:10.1073/pnas.0701100104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- deYoung B., et al. 2008Regime shifts in marine ecosystems: detection, prediction and management. Trends Ecol. Evol. 23, 402–409 (doi:10.1016/j.tree.2008.03.008) [DOI] [PubMed] [Google Scholar]
- Frank K. T., Petrie B., Choi J. S., Leggett W. C.2005Trophic cascades in a formerly cod-dominated ecosystem. Science 308, 1621–1623 (doi:10.1126/science.1113075) [DOI] [PubMed] [Google Scholar]
- Heath M. R.2005Changes in the structure and function of the North Sea fish foodweb, 1973–2000, and the impacts of fishing and climate. ICES J. Mar. Sci. 62, 847–868 (doi:10.1016/j.icesjms.2005.01.023) [Google Scholar]
- Hsieh C. H., Glaser S. M., Lucas A. J., Sugihara G.2005Distinguishing random environmental fluctuations from ecological catastrophes for the North Pacific Ocean. Nature 435, 336–340 (doi:10.1038/nature03553) [DOI] [PubMed] [Google Scholar]
- Hughes T. P., Bellwood D. R., Folke C., Steneck R. S., Wilson J.2005New paradigms for supporting the resilience of marine ecosystems. Trends Ecol. Evol. 20, 380–386 (doi:10.1016/j.tree.2005.03.022) [DOI] [PubMed] [Google Scholar]
- Ibañez F., Etienne M.1992Le filtrage des séries chronologiques par l'analyse en composantes principales de processus (ACPP). J. Rech. Océanogr. 16, 27–33 [Google Scholar]
- ICES 2006Report of the ICES advisory committee on fishery management, advisory committee on the marine environment and advisory committee on ecosystems, 2006. ICES Advice; Books 1–10. 6: 310pp Copenhagen, Denmark: ICES [Google Scholar]
- IPCC 2007Climate change 2007: impacts, adaptation and vulnerability Cambridge, UK: Cambridge University Press [Google Scholar]
- Jackson J. B. C.2008Ecological extinction and evolution in the brave new ocean. Proc. Natl Acad. Sci. USA 105, 11 458–11 465 (doi:10.1073/pnas.0802812105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby R. R., Beaugrand G., Lindley J. A.2008Climate-induced effects on the merozooplankton and the benthic–pelagic ecology of the North Sea. Limnol. Oceanogr. 53, 1805–1815 [Google Scholar]
- Kirby R. R., Beaugrand G., Lindley J. A.2009Synergistic effects of climate and fishing in a marine ecosystem. Ecosystems 12, 548–561 (doi:10.1007/s10021-009-9241-9) [Google Scholar]
- Kirby R. R., et al. 2007Climate effects and benthic–pelagic coupling in the North Sea. Mar. Ecol. Prog. Ser. 330, 31–38 (doi:10.3354/meps330031) [Google Scholar]
- Legendre P., Legendre P.1998Numerical ecology: developments in environmental modeling 20 Amsterdam, The Netherlands: Elsevier Science BV [Google Scholar]
- Lindley J. A., Williams R., Hunt H. G.1993Anomalous seasonal cycles of decapod crustacean larvae in the North Sea in an exceptionally warm year. J. Exp. Mar. Biol. Ecol. 172, 47–66 (doi:10.1016/0022-0981(93)90088-6) [Google Scholar]
- Longhurst A.2006Viewpoint—The sustainability myth. Fish. Res. 81, 107–112 (doi:10.1016/j.fishres.2006.06.022) [Google Scholar]
- Lovelock J. E.1979Gaia, a new look at life of Earth Oxford, UK: Oxford University Press [Google Scholar]
- MacKenzie B. R., Myers R. A.2007The development of the northern European fishery for north Atlantic bluefin tuna, Thunnus thynnus during 1900–1950. Fisheries Res. 87, 229–239 [Google Scholar]
- Molinero J. C., Ibanez F., Souissi S., Chiffet M., Nival P.2005Phenological changes in the northwestern Mediterranean copepods Centropages typicus and Temora stylifera linked to climate forcing. Oecologia 145, 640–649 (doi:10.1007/s00442-005-0130-4) [DOI] [PubMed] [Google Scholar]
- Muradian R.2001Ecological thresholds: a survey. Ecol. Econ. 38, 7–24 (doi:10.1016/S0921-8009(01)00146-X) [Google Scholar]
- Oguz T.2007Nonlinear response of Black Sea pelagic fish stocks to over-exploitation. Mar. Ecol. Prog. Ser. 34, 211–228 [Google Scholar]
- Österblom H., et al. 2007Human-induced trophic cascades and ecological regime shifts in the Baltic Sea. Ecosystems 10, 877–889 (doi:10.1007/s10021-007-9069-0) [Google Scholar]
- Pace M. L., Cole J. J., Carpenter S. R., Kitchell J. F.1999Trophic cascades revealed in diverse ecosystems. Trends Ecol. Evol. 14, 483–488 (doi:10.1016/S0169-5347(99)01723-1) [DOI] [PubMed] [Google Scholar]
- Pauly D., Christensen V., Dalsgaard J., Froese R., Torres F.1998Fishing down marine food webs. Science 279, 860–863 (doi:10.1126/science.279.5352.860) [DOI] [PubMed] [Google Scholar]
- Pikitch E. K., et al. 2004Ecosystem-based fishery management. Science 305, 346–347 (doi:10.1126/science.1098222) [DOI] [PubMed] [Google Scholar]
- Raitsos D. E., Reid P. C., Lavender S. J., Edwards M., Richardson A. J.2005Extending the SeaWIFS chlorophyll dataset back 50 years in the northeast Atlantic. Geophys. Res. Lett. 32, L06603 (doi:10.1029/2005GL022484) [Google Scholar]
- Rees C. B.1955Continuous plankton records: the decapod larvae in the North Sea 1950–51. Bull. Mar. Ecol. 4, 69–80 [Google Scholar]
- Rees H. L., Eggleton J. D., Rachor E., Vanden Berghe E.2007Structure and dynamics of the North Sea benthos. ICES Cooperative Research Report, 288. Copenhagen, Denmark: ICES [Google Scholar]
- Scheffer M., Carpenter S. R.2003Catastrophic regime shifts in ecosystems: linking theory to observation. Trends Ecol. Evol. 18, 648–656 (doi:10.1016/j.tree.2003.09.002) [Google Scholar]
- Scheffer M., Carpenter S., deYoung B.2005Cascading effects of overfishing marine ecosystems. Trends Ecol. Evol. 20, 579–581 (doi:10.1016/j.tree.2005.08.018) [DOI] [PubMed] [Google Scholar]
- Stige L. C., et al. 2009Climatic forcing of zooplankton dynamics is stronger during low densities of planktivorous fish. Limnol. Oceanogr. 54, 1025–1036 [Google Scholar]
- Taylor A. H., Allen J. I., Clark P. A.2002Extraction of a weak climatic signal by an ecosystem. Nature 416, 629–632 (doi:10.1038/416629a) [DOI] [PubMed] [Google Scholar]
- Travers M., Shin Y.-J., Jennings S., Cury P.2007Towards end-to-end models for investigating the effects of climate and fishing in marine ecosystems. Prog. Oceanogr. 75, 751–770 (doi:10.1016/j.pocean.2007.08.001) [Google Scholar]
- van der Veer H., Bergman J. N.1987Predation by crustaceans on a newly settled 0-group plaice Pleuronectes platessa population in the western Wadden Sea. Mar. Ecol. Prog. Ser. 35, 203–215 (doi:10.3354/meps035203) [Google Scholar]
- Vautard R. P. Y., Ghil M.2002Singular-spectrum analysis: a toolkit for short, noisy chaotic signals. Physica D 58, 95–126 (doi:10.1016/0167-2789(92)90103-T) [Google Scholar]
- Weijerman M., Lindeboom H. J., Zuur A.2005Regime shifts in marine ecosystems of the North Sea and Wadden Sea. Mar. Ecol. Prog. Ser. 289, 21–39 [Google Scholar]
- Woodruff S. D., Diaz H. F., Worley S. J.1988COADS release 2 data and metadata enhancements for improvements of marine surface flux fields. Phys. Chem. Earth 25, 517–527 [Google Scholar]