Abstract
Sexual selection theory for separate-sexed animals predicts that the sexes differ in the benefit they can obtain from multiple mating. Conventional sex roles assume that the relationship between the number of mates and the fitness of an individual is steeper in males compared with females. Under these conditions, males are expected to be more eager to mate, whereas females are expected to be choosier. Here we hypothesize that the sex allocation, i.e. the reproductive investment devoted to the male versus female function, can be an important predictor of the mating strategy in simultaneous hermaphrodites. We argue that within-species variation in sex allocation can cause differences in the proportional fitness gain derived through each sex function. Individuals should therefore adjust their mating strategy in a way that is more beneficial to the sex function that is relatively more pronounced. To test this, we experimentally manipulated the sex allocation in a simultaneously hermaphroditic flatworm and investigated whether this affects the mating behaviour. The results demonstrate that individuals with a more male-biased sex allocation (i.e. relatively large testes and small ovaries) are more eager to mate compared with individuals with a more female-biased sex allocation (i.e. relatively small testes and large ovaries). We argue that this pattern is comparable to conventional gender roles in separate-sexed organisms.
Keywords: gender role, Macrostomum lignano, mating behaviour, ovary size, phenotypic plasticity, testis size
1. Introduction
Darwin was puzzled by the observation that throughout the animal kingdom the female seems to be ‘less eager than the male’ and suggested that the ‘exertion of some choice on the part of the female seems a law almost as general as the eagerness of the male’ (Darwin 1871). The most cited explanation for this observation is Bateman's principle. Based on mating experiments with Drosophila, Bateman (1948) argued that the relationship between reproductive success and the number of mates is steeper in males than in females. Consequently, males gain more from mating with different partners and thus should show an ‘undiscriminating eagerness’, whereas females are expected to display a ‘discriminating passivity’ (Bateman 1948). Although there is an ongoing debate on the validity of Bateman's original study and its implications (e.g. Sutherland 1985; Snyder & Gowaty 2007; Jones 2009), it nevertheless holds that in many animal species (including humans) males and females differ in the benefit they derive from an elevated mating success (e.g. Jones et al. 2000, 2002; Brown et al. 2009).
No such general statement can be made for the male and female functions of simultaneously hermaphroditic animals (i.e. organisms that produce sperm and eggs at the same time). Here copulations often occur reciprocally (i.e. both mating partners donate and receive sperm; Charnov 1979; Michiels 1998; Anthes et al. 2006) so that the mating rate of one sex function is directly linked to that of the other. On the assumption that the male and the female functions have different optimal mating rates (Anthes et al. 2006), a trade-off between two mating strategies within an individual could result, for example being eager to mate in one function versus being choosy in the other function (at least at the pre-copulatory stage).
Recent research on mating strategies in simultaneous hermaphrodites primarily asked whether individuals of a species have an overall preference for mating in the male role (i.e. to donate sperm in order to fertilize eggs) or the female role (i.e. to receive sperm in order to get the own eggs fertilized and/or to benefit from sperm digestion; reviewed in Michiels 1998; Anthes et al. 2006). However, theoretical studies suggest that the preferred sex role can also be flexible within a species and predict that the preference to mate in one sex function may depend on factors such as body size, the quality of the partner, the sperm precedence pattern and the mating history of available mates (Angeloni et al. 2002; Anthes et al. 2006). In particular, it has been proposed that the preferred sex role of an individual should depend on the relation of its own body size to that of the partner (Angeloni et al. 2002) and there is empirical evidence that small individuals mate preferentially in the male reproductive role whereas large individuals prefer the female role (e.g. Ohbayashi-Hodoki et al. 2004; Norton et al. 2008; but see Gianguzza et al. 2004).
A widely unexplored parameter that might have a direct effect on the preferred mating strategy of an individual is its own sex allocation (i.e. the allocation of resources towards the male versus the female function). Schärer (2009) suggested that between-species variation in sex role preferences might be explained by differences in the average sex allocation. Based on the Fisher condition (Houston & McNamara 2002), the overall fitness return per unit investment is expected to be higher for the sex function with the lower allocation. Therefore, in species with an overall female-biased sex allocation, individuals should prefer to mate in the male function (Schärer 2009). Beyond that, sex allocation might also explain variation in sex role preferences within a species. The only study providing correlational support for the link between sex allocation and mating behaviour within a species was carried out on blue-banded gobies (Lythrypus dalli), showing that egg-laying individuals had a more female-biased sex allocation compared with those individuals that fertilized eggs (St Mary 1994). However, theoretical and experimental work focusing on the effect of sex allocation on mating rates in simultaneous hermaphrodites is lacking.
Here we propose that within a species the sex allocation directly affects the mating strategy independently of body size, and we provide the first experimental evidence in support of this hypothesis. In many simultaneously hermaphroditic animals, there is considerable within-species variation in sex allocation (for review, see Schärer 2009). Owing to this variation, individuals are expected to differ in the proportional fitness gains they derive from either sex function (Charnov 1982). When we now assume that both sex functions differ in their benefit obtained from a higher mating success, we hypothesize that the sex allocation has an effect on the mating rate an individual exhibits. Whether a more male- or female-biased sex allocation leads to a higher mating rate depends on which sex function has a higher benefit of multiple mating, which brings us back to Bateman's principle.
Charnov (1979) argued that Bateman's principle also applies to hermaphrodites and hypothesized that ‘individuals copulate not so much to gain sperm to fertilize eggs as to give sperm away’. In contrast, it has also been claimed that it is the female role that should be preferred during mating, since the female function has a lower risk of a total reproductive failure (Leonard 2005). If the relationship between reproductive success and mating success (i.e. the Bateman gradient) is steeper for the male function, one would expect that the reproductive success of more male-biased individuals depends more on the number of mates compared to more female-biased individuals. Therefore, more male-biased individuals should be more eager to mate, so that their increased investment into their male function pays off. Conversely, individuals with a more female-biased sex allocation should adopt a mating strategy that is more discriminating and that selects for mating partners that provide the highest direct or indirect benefits. In contrast, if the Bateman gradient is steeper for the female function, we expect that more female-biased individuals gain more from multiple mating and should therefore be more prone to mate.
Here we compared the mating rates of pairs that were either formed by joining two more male-biased individuals or two more female-biased individuals of the outcrossing simultaneously hermaphroditic flatworm Macrostomum lignano. In these worms, sex allocation is phenotypically plastic and adjusted in response the number of potential mates (i.e. the social group size; e.g. Schärer & Ladurner 2003; Brauer et al. 2007; Schärer & Janicke in press). This offers the opportunity to manipulate the sex allocation experimentally and to test how this affects the mating behaviour. If M. lignano mates primarily in order to donate sperm, as predicted by sexual selection theory for simultaneous hermaphrodites (Charnov 1979), we expect that more male-biased individuals show higher mating rates. In addition, we tested whether sex allocation also influences other aspects of the mating behaviour, namely the copulation duration and the frequency of a post-copulatory behaviour.
2. Material and Methods
(a). Study organism
Macrostomum lignano (Macrostomorpha, Platyhelminthes) is a simultaneously hermaphroditic free-living flatworm of the intertidal meiofauna of the Northern Adriatic Sea (Ladurner et al. 2005). In culture, it reaches 1.5 mm in body length and has a generation time of about 18 days. In mass cultures, worms are maintained at 20°C in glass Petri dishes containing f/2 medium (Andersen et al. 2005) and fed with the diatom Nitzschia curvilineata. The worm is fairly transparent, allowing non-invasive measurement of morphological traits such as body size, testis size, ovary size and seminal vesicle size (Schärer & Ladurner 2003). The seminal vesicle represents the sperm storage organ of the male function and is located in the tail plate of the worm. A previous study showed that the size of the seminal vesicle is a good proxy for the number of sperm it contains (Schärer & Vizoso 2007).
Mating is reciprocal and often accompanied by a post-copulatory ‘suck’ in which the worm bends itself in order to touch its own female genital opening with the pharynx (Schärer et al. 2004). After this behaviour, a bundle of sperm often sticks out of the worm's own female genital opening, suggesting that the recipient may suck some of the received ejaculate out of its female sperm storage organ. Therefore, this behaviour may represent a mechanism to select among sperm from different sperm donors (i.e. cryptic female choice; sensu Thornhill 1983). By sucking, worms may remove unfavoured ejaculates out of their sperm storage organ and/or enable favoured sperm to get anchored in a part of a tissue that is most likely to lead to fertilization. Moreover, sucking sperm might also prevent costs associated with polyspermy (Arnqvist & Rowe 2005). However, until now there is no clear evidence showing that this behaviour is actually linked to post-copulatory female choice.
(b). Manipulation of sex allocation
To test whether sex allocation has an effect on the mating rate, we manipulated the sex allocation of our focal worms prior to the mating trials using the approach outlined in the introduction (i.e. by raising worms in different social groups). On day 1 we collected 1200 adult worms and distributed them equally to 12 Petri dishes filled with f/2 medium and a dense layer of algae where they could lay eggs. After 48 h we removed all adult worms, limiting the difference in laying date to 2 days. On day 11 we pooled all resulting hatchlings and distributed them randomly into their different social group sizes, namely pairs and octets (i.e. groups of two or eight individuals, respectively), into wells of 24-hole well plates. The treatments were arranged to balance any possible position effects (i.e. always two replicates of both treatments per plate and positions on the plate alternated). Wells were filled with 2 ml of f/2 medium and a dense algae suspension that guaranteed ad libitum food conditions. On days 21, 28 and 35 we transferred only adult worms to fresh wells. With this setup, we ensured that the manipulated social group size was not influenced by the produced offspring, because worms usually hatch after 5 days after egg laying and do not mature until 13 days after hatching (Schärer & Ladurner 2003). Each treatment was replicated 54 times.
(c). Morphological measurement of sex allocation
To check whether the treatment successfully manipulated the sex allocation of the worms, we took images for morphological measurements in vivo prior to the mating trials. This was done by compressing anaesthetized worms dorsoventrally to a fixed thickness of 35 µm between a microscope slide and a coverslip of a haemocytometer (Schärer & Ladurner 2003). Image acquisition was carried out from days 36 to 41. We used a Leica DM 2500 microscope (Leica Microsystems, Germany) to which we connected a digital video camera (DFK 41BF02, The Imaging Source Europe GmbH, Bremen, Germany) and took digital micrographs at 40× for body size and 400× for gonad size and seminal vesicle size. Recent studies have shown for M. lignano that testis size is a good proxy for sperm production rate (Schärer & Vizoso 2007) and that ovary size covaries positively with female fecundity (P. Sandner 2005, unpublished data). For image acquisition, we used the software BTV Pro 6.0b1 (http://www.bensoftware.com/) and we analysed micrographs using Image 1.38x (http://rsb.info.nih.gov/ij/). The time period between imaging and the start of the mating trials was on average 140.3 ± 7.9 min (mean ± s.e.) and did not differ between the treatment groups (t-test: t106 = 0.50, p = 0.640).
(d). Mating trials and quantification of mating behaviour
From days 36 to 41 we filmed the mating behaviour of pairs formed by joining individuals that originated from the social group size treatments described above. In particular, these mating pairs were composed of two individuals selected randomly either from two different pairs (hereinafter called ‘Ps’) or from two different octets (hereinafter called ‘Os’). Consequently, we offered to all individuals one mating partner that had experienced the same social group size before, but which came from a different replicate. By using only one worm from each replicate, we ensured that all mating pairs were completely independent.
We conducted mating trials in observation chambers as described in detail elsewhere (Schärer et al. 2004). We placed two worms in a drop of 6 µl artificial sea water (salinity of 32‰) between two microscope slides. In these drops, worms seem to behave similarly as they do in mass cultures but are somewhat restricted into two dimensions, which allows a better observation and quantification of the mating behaviour. Mating trials lasted 2 h during which no food was provided.
In total, we assembled 17 chambers each with two or four mating pairs. For each chamber, we balanced the number of treatments (i.e. 10 chambers with two mating pairs of each treatment and 7 chambers with one mating pair of each treatment) and we alternated the positions of the drops within the chamber to avoid any position effects.
We filmed each chamber for 2 h at 1 frame s−1 using a digital video camera (DFK 31BF03, The Imaging Source Europe GmbH) and recorded movies in QuickTime format using BTV Pro 5.4.1. (http://www.bensoftware.com/). Movie capture started within 5 min after chamber assembly. We scored mating movies using BTV Pro 6.0b1. By manual frame-by-frame analysis of the QuickTime movies, we assessed for the entire observation period of 2 h the following parameters: the number of copulations, the average copulation duration and the number of sucks.
(e). Statistical analyses
Of 54 mating pairs that we filmed, seven were excluded from the analyses. Specifically, in three cases, one individual of the mating pair lacked the seminal vesicle or the male copulatory organ (a condition that can be found rarely but regularly in lab cultures but also in field-caught worms), two replicates were excluded because of pipetting errors, one because one worm was injured, and one because one worm was not mature. The final dataset comprised 47 mating pairs (24 Ps and 23 Os). For both individuals within each mating pair, we measured all morphological traits, except for one individual for which it was not possible to get accurate photographs of its ovaries.
To test the effect of social group size on morphological traits, we used general linear models with social group size as a fixed factor and body size as a covariate, since gonad size is usually positively correlated with body size in M. lignano (e.g. Schärer & Ladurner 2003). As a combined measure of the resource allocation towards the male versus the female function, we defined sex allocation as testis size divided by the sum of testis and ovary size (Vizoso & Schärer 2007). Sex allocation thus represents a relative measure that allows comparing the resource allocation between individuals. However, it does not represent an absolute measure of the sex allocation since it does not account for potential differences in the energy demand per unit of testicular and ovarian tissue (for details, see Schärer 2009). By definition, individuals with a relatively high sex allocation are considered more male-biased. Statistical comparisons of behavioural parameters were done using two-sample t-tests. Copulation duration refers to the mean value over all copulations recorded within a mating pair. All statistics were carried out using SPSS 13.0 (SPSS Inc., Chicago, IL, USA) or JMP 7.0.1. (SAS Institute Inc., Cary, NC, USA). Values are given as means ± s.e. unless otherwise stated.
3. Results
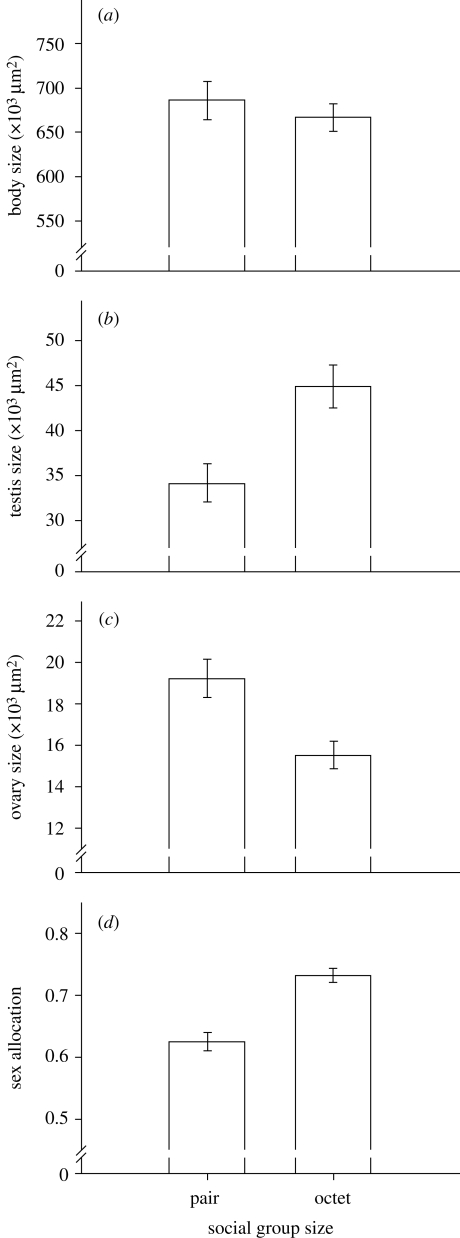
Exposing worms to different social group sizes had no effect on body size (Welch's t-test: t84.6 = 0.72, p = 0.473; figure 1a; electronic supplementary material, table S1), which suggests that worms grew equally well in both treatments. Overall, body size was positively related to ovary size and showed a strong trend to predict variation in testis size (table 1). However, there was no relationship between body size and measures of sex allocation and seminal vesicle size (table 1).
Figure 1.
Effect of social group size on sex allocation in Macrostomum lignano. Differences in (a) body size, (b) testis size, (c) ovary size and (d) sex allocation are shown for individuals that were raised in pairs and octets. Sex allocation refers to testis size divided by the sum of testis size and ovary size. Bars indicate means ± s.e.
Table 1.
General linear models testing the effect of the social group size and body size on morphological parameters. Residuals of all models did not deviate significantly from a normal distribution (Kolmogorov–Smirnov, all p > 0.05).
| morphological parameter | model fit |
factor: social group size |
covariate: body size |
||||
|---|---|---|---|---|---|---|---|
| r2 | F | d.f. | p-value | F | d.f. | p-value | |
| testis size | 0.15 | 13.7 | 1,91 | <0.001 | 3.8 | 1,91 | 0.053 |
| ovary size | 0.16 | 9.7 | 1,90 | 0.002 | 6.1 | 1,90 | 0.016 |
| sex allocation | 0.27 | 33.9 | 1,90 | <0.001 | 0.7 | 1,90 | 0.407 |
| seminal vesicle size | 0.14 | 14.3 | 1,91 | <0.001 | 0.4 | 1,91 | 0.514 |
Social group size had a strong effect on sex allocation. Worms that originated from octets had larger testes, smaller ovaries and consequently a higher sex allocation (table 1; figure 1; electronic supplementary material, table S1). Worms kept in octets had 31.7 per cent larger testes but 19.2 per cent smaller ovaries compared with worms that were kept in pairs. Therefore, worms from octets were clearly more male-biased compared with worms from pairs. In addition, the size of the seminal vesicle was smaller in individuals originating from octets (table 1; electronic supplementary material, table S1).
Of the 47 mating pairs that we observed, five did not copulate during the mating trials (four Ps and one O). The number of sucks was highly correlated with the number of copulations (Spearman's ρ = 0.83, p < 0.001, n = 47). Therefore, we used the number of sucks divided by the number of copulations as a relative measure of the frequency of the suck behaviour. The number of copulations, the average copulation duration and the relative number of sucks were not correlated among each other (Spearman; all p > 0.1, n = 42).
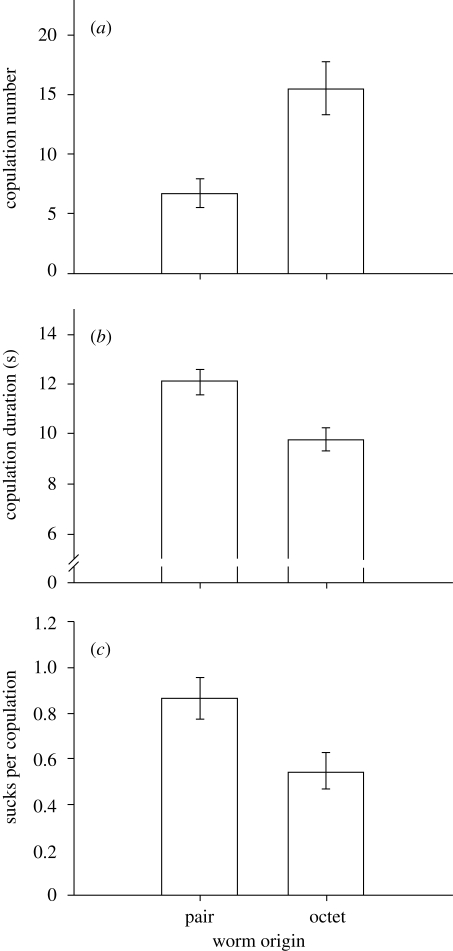
Individuals that differed in their original social environment and consequently in their sex allocation behaved differently with respect to all behavioural parameters we tested. In particular, Os (more male-biased worms) copulated more than twice as often compared with Ps (more female-biased worms; table 2; figure 2a). Even when excluding mating pairs that did not copulate at all (n = 5), this difference remained significant (number of copulations: Ps: 8.1 ± 1.8, Os: 16.2 ± 1.8; Welch's t-test: t33.2 = −3.21, p = 0.003). Overall, there was also a positive correlation between the average sex allocation of both mating partners and their copulation number (Spearman's ρ = 0.36, p = 0.014, n = 47), but this relationship was not significant within both treatments (within pairs: Pearson, r = −0.18, p = 0.397, n = 24; within octets: Spearman's ρ = 0.28, p = 0.193, n = 23).
Table 2.
Comparison of the mating behaviour between worms that originated from pairs (more female-biased individuals) and worms that originated from octets (more male-biased individuals). Statistics refer to Student's t-tests. For the comparison of copulation number, the Welch's t-test was used since variances were not equal.
| behavioural parameter | pair |
octet |
t | d.f. | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| mean ± s.e. | min | max | mean ± s.e. | min | max | ||||
| copulation number | 6.7 ± 1.2 | 0 | 27 | 15.5 ± 2.2 | 0 | 44 | −3.47 | 34.3 | 0.001 |
| copulation duration (s) | 12.1 ± 0.5 | 8.8 | 17.1 | 9.8 ± 0.5 | 7 | 14.4 | 3.43 | 40 | 0.001 |
| sucks per copulation | 0.9 ± 0.1 | 0.2 | 1.7 | 0.5 ± 0.1 | 0 | 1.5 | 2.62 | 40 | 0.013 |
Figure 2.
Comparison of mating behaviour between more female-biased pairs (both individuals originated from pairs) and more male-biased pairs (both individuals originated from octets). Means for (a) copulation number, (b) the average copulation duration and (c) the relative number of sucks (i.e. corrected for the number of copulations) are depicted from mating trials that lasted 2 h. Bars indicate means ± s.e.
There was also a difference in copulation duration. Copulations in Ps lasted longer than copulations in Os (table 2; figure 2b). Furthermore, we found an effect of social group size on the relative number of sucks, which was higher in Ps compared with Os (table 2; figure 2c).
4. Discussion
In this study, we (i) confirm that the social group size has an effect on the sex allocation in a simultaneous hermaphrodite, as predicted by sex allocation theory, and (ii) demonstrate for the first time that this manipulation leads to changes in the mating behaviour. We thus provide the first experimental evidence that more male-biased simultaneous hermaphrodites show an increased mating rate, which is consistent with the view that simultaneous hermaphrodites mate primarily in order to donate sperm (Charnov 1979). We discuss these two findings in turn.
(a). Social group size and reproductive morphology
Worms that were raised in octets had larger testes and smaller ovaries compared with individuals that were raised in pairs. This finding confirms earlier studies on M. lignano and other simultaneously hermaphroditic animals showing that individuals in larger social groups invest relatively more reproductive resources into the male function (reviewed in Schärer 2009) as predicted by sex allocation theory (Charnov 1982). More importantly, our data represent one of the clearest examples of a trade-off between the reproductive investment to the male versus female function, which is a fundamental (Charnov 1982), but poorly supported, assumption of sex allocation theory for simultaneous hermaphrodites (reviewed in Schärer 2009). A previous study also found a trade-off in sex allocation in M. lignano, but there the trade-off was only visible under specific conditions and the sample size was relatively small (Schärer et al. 2005). We are aware of only two other empirical studies that also support the existence of a trade-off between male and female allocation in simultaneous hermaphrodites (De Visser et al. 1994; Yund et al. 1997).
In our experiment, social group size had no significant effect on body size so that the final test of the effect of social group size on mating behaviour is not confounded by differences in body size. However, there was a difference in seminal vesicle size between the two treatments. Worms from octets had smaller seminal vesicles compared with worms from pairs, which corresponds to previous findings (Schärer & Ladurner 2003; Brauer et al. 2007). Given that the size of the seminal vesicle correlates with the number of sperm it contains in M. lignano (Schärer & Vizoso 2007), individuals in octets presumably transferred more sperm and had consequently smaller seminal vesicles than individuals that were raised in pairs.
(b). Sex allocation and mating behaviour
The main aim of this study was to test whether sex allocation has an effect on the mating rate. Our results show that individuals that differ in their sex allocation behave differently. Pairs formed by two individuals that had a more male-biased sex allocation copulated more than twice as much compared with pairs formed by more female-biased partners. This suggests that the sex allocation of a simultaneous hermaphrodite can be a strong predictor of the mating strategy an individual adopts. Our result corresponds to a previous study in M. lignano that showed that testis size is positively correlated with the number of mating partners at a given group size (Janicke & Schärer 2009). Similar to the conventional gender roles in gonochorists, it appears that more male-biased individuals are relatively more eager to mate, whereas more female-biased individuals are more reluctant. Therefore, we speculate that an elevated mating rate is more beneficial to the male than to the female function.
As we did not manipulate sex allocation directly, it is possible that factors other than sex allocation could have caused the observed effects on mating rate. First, there might have been carry-over effects in our experimental setup. In particular, worms from octets may have copulated more frequently in the mating trials because they were used to mate more often due to higher encounter rates and/or higher levels of sperm competition in their originally larger social groups. This could explain why we failed to show a positive correlation between sex allocation and mating rate within both treatments. However, this lack could also be due to a relatively low variation in sex allocation within the treatments, which makes it more difficult to detect such a correlation. Furthermore, a previous study on the effect of social group size on sex allocation in M. lignano showed that the size of the seminal vesicle decreases within 24 h after transferring a worm from a pair into an octet (Brauer et al. 2007). The authors interpreted this result as a direct adjustment of the mating rate to different social group sizes. However, the observed decrease in the seminal vesicle size in octets does not necessarily have to reflect a change in the mating rate but could also be caused by an increased sperm allocation per mating as predicted by sperm competition theory (for review, see Wedell et al. 2002).
Second, raising worms in different social group sizes also had an effect on the size of the seminal vesicle, and therefore the amount of sperm that was available during mating (Schärer & Vizoso 2007). Assuming that the number of available sperm affected the mating rate, it would be more intuitive to predict that individuals with larger sperm reserves copulate more frequently. However, our results indicate the opposite, namely that the worms with less sperm to allocate were more eager to mate. Therefore, it seems unlikely that the size of the seminal vesicle can explain the higher mating rate in worms from octets that we observed in our experiment.
Besides the effect on mating rate, we also observed a difference in copulation duration among our treatments. Pairs of more male-biased worms copulated more briefly compared with more female-biased pairs. For many species, it has been shown that copulation duration correlates with the number of transferred sperm (e.g. Engqvist & Sauer 2003) and this is the case in M. lignano (P. Sandner 2009, personal communication). Therefore, we suppose that the reduced copulation duration we found was an effect of the smaller seminal vesicle size of more male-biased individuals rather than a consequence of an increased sex allocation per se.
Additionally, individuals that originated from pairs and octets also differed in the relative number of the sucks. Interestingly, worms that were raised in pairs that copulated with a new partner sucked more often (relative to the number of copulations) compared with worms that were raised in octets that also mated with a new partner. On the assumption that the suck behaviour is a form of cryptic female choice (see §1) and that Bateman's principle applies, one would expect worms that invest more into the female function to be choosier and therefore suck more often. This corresponds exactly to the pattern we have found. However, worms from pairs also had larger seminal vesicles and might have received more sperm from their mate during the mating trials. Therefore, individuals in pairs may have sucked more frequently only to remove surplus ejaculate without discriminating among sperm donors.
To conclude, our study demonstrates that the sex allocation might help to explain inter-individual variation in mating strategies of simultaneous hermaphrodites. We show that individuals with a more male-biased sex allocation copulate more frequently, which corresponds to conventional gender roles in separate-sexed animals. There is a clear need for more empirical and theoretical studies investigating how sex allocation affects the mating strategies and gender roles of simultaneous hermaphrodites. Particularly, the causal relationship between sex allocation and mating rate requires further testing. Future experiments might investigate existing variation in sex allocation among individuals that were exposed to the same conditions to test whether sex allocation correlates with various aspects of the mating behaviour.
Acknowledgements
We thank Nils Anthes, Ralph Dobler, Peter Sandner and two anonymous referees for constructive comments on an earlier version of the manuscript. Jürgen Hottinger, Viktor Mislin and Urs Stiefel kindly provided technical support. This project was funded by the Swiss National Science Foundation (3100A0-113708).
References
- Andersen R. A., Berges J. A., Harrison P. J., Watanabe M. M.2005Recipes for freshwater and seawater media. In Algal culturing techniques (ed. Andersen R. A.), pp. 429–538 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- Angeloni L., Bradbury J. W., Charnov E. L.2002Body size and sex allocation in simultaneously hermaphroditic animals. Behav. Ecol. 13, 419–426 (doi:10.1093/beheco/13.3.419) [Google Scholar]
- Anthes N., Putz A., Michiels N. K.2006Sex role preferences, gender conflict and sperm trading in simultaneous hermaphrodites: a new framework. Anim. Behav. 72, 1–12 (doi:10.1016/j.anbehav.2005.09.017) [Google Scholar]
- Arnqvist G., Rowe L.2005Sexual conflict. Monographs in Behaviour and Ecology. Princeton, NJ: Princeton University Press [Google Scholar]
- Bateman A. J.1948Intra-sexual selection in Drosophila. Heredity 2, 349–368 (doi:10.1038/hdy.1948.21) [DOI] [PubMed] [Google Scholar]
- Brauer V. S., Schärer L., Michiels N. K.2007Phenotypically flexible sex allocation in a simultaneous hermaphrodite. Evolution 61, 216–222 (doi:10.1111/j.1558-5646.2007.00018.x) [DOI] [PubMed] [Google Scholar]
- Brown G. R., Laland K. N., Borgerhoff Mulder M.2009Bateman's principles and human sex roles. Trends Ecol. Evol. 24, 297–304 (doi:10.1016/j.tree.2009.02.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnov E. L.1979Simultaneous hermaphroditism and sexual selection. Proc. Natl Acad. Sci. USA 76, 2480–2484 (doi:10.1073/pnas.76.5.2480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnov E. L.1982The theory of sex allocation. Monographs in Population Biology. Princeton, NJ: Princeton University Press; [PubMed] [Google Scholar]
- Darwin C. R.1871The descent of man, and selection in relation to sex London, UK: John Murray [Google Scholar]
- De Visser J. A. G. M., Termaat A., Zonneveld C.1994Energy budgets and reproductive allocation in the simultaneous hermaphrodite pond snail, Lymnaea stagnalis (L)—a trade-off between male and female function. Am. Nat. 144, 861–867 [Google Scholar]
- Engqvist L., Sauer K. P.2003Determinants of sperm transfer in the scorpionfly Panorpa cognata: male variation, female condition and copulation duration. J. Evol. Biol. 16, 1196–1204 (doi:10.1046/j.1420-9101.2003.00613.x) [DOI] [PubMed] [Google Scholar]
- Gianguzza P., Badalamenti F., Jensen K. R., Chemello R., Cannicci S., Riggio S.2004Body size and mating strategies in the simultaneous hermaphrodite Oxynoe olivacea (Mollusca, Opisthobranchia, Sacoglossa). Funct. Ecol. 18, 899–906 (doi:10.1111/j.0269-8463.2004.00911.x) [Google Scholar]
- Houston A. I., McNamara J. M.2002A self-consistent approach to paternity and parental effort. Phil. Trans. R. Soc. Lond. B 357, 351–362 (doi:10.1098/rstb.2001.0925) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke T., Schärer L.2009Determinants of mating and sperm-transfer success in a simultaneous hermaphrodite. J. Evol. Biol. 22, 405–415 (doi:10.1111/j.1420-9101.2008.01660.x) [DOI] [PubMed] [Google Scholar]
- Jones A. G.2009On the opportunity for sexual selection, the Bateman gradient and the maximum intensity of sexual selection. Evolution 63, 1673–1684 (doi:10.1111/j.1558-5646.2009.00664.x) [DOI] [PubMed] [Google Scholar]
- Jones A. G., Rosenqvist G., Berglund A., Arnold S. J., Avise J. C.2000The Bateman gradient and the cause of sexual selection in a sex-role-reversed pipefish. Proc. R. Soc. Lond. B 267, 677–680 (doi:10.1098/rspb.2000.1055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. G., Arguello J. R., Arnold S. J.2002Validation of Bateman's principles: a genetic study of sexual selection and mating patterns in the rough-skinned newt. Proc. R. Soc. Lond. B 269, 2533–2539 (doi:10.1098/rspb.2002.2177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladurner P., Schärer L., Salvenmoser W., Rieger R. M.2005A new model organism among the lower Bilateria and the use of digital microscopy in taxonomy of meiobenthic Platyhelminthes: Macrostomum lignano, n. sp. (Rhabditophora, Macrostomorpha). J. Zool. Syst. Evol. Res. 43, 114–126 (doi:10.1111/j.1439-0469.2005.00299.x) [Google Scholar]
- Leonard J. L.2005Bateman's principle and simultaneous hermaphrodites: a paradox. Integr. Comp. Biol. 45, 856–873 (doi:10.1093/icb/45.5.856) [DOI] [PubMed] [Google Scholar]
- Michiels N. K.1998Mating conflicts and sperm competition in simultaneous hermaphrodites. In Sperm competition and sexual selection (eds Birkhead T., Møller A. P.), pp. 219–254 London, UK: Academic Press [Google Scholar]
- Norton C. G., Johnson A. F., Mueller R. L.2008Relative size influences gender role in the freshwater hermaphroditic snail, Helisoma trivolvis. Behav. Ecol. 19, 1122–1127 (doi:10.1093/beheco/arn099) [Google Scholar]
- Ohbayashi-Hodoki K., Ishihama F., Shimada M.2004Body size-dependent gender role in a simultaneous hermaphrodite freshwater snail, Physa acuta. Behav. Ecol. 15, 976–981 (doi:10.1093/beheco/arh101) [Google Scholar]
- Schärer L.2009Tests of sex allocation theory in simultaneously hermaphroditic animals. Evolution 63, 1377–1405 (doi:10.1111/j.1558-5646.2009.00669.x) [DOI] [PubMed] [Google Scholar]
- Schärer L., Janicke T.In press Sex allocation and sexual conflict in simultaneously hermaphroditic animals. Biol. Lett (doi:10.1098/rspb.2009.0100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schärer L., Ladurner P.2003Phenotypically plastic adjustment of sex allocation in a simultaneous hermaphrodite. Proc. R. Soc. Lond. B 270, 935–941 (doi:10.1098/rspb.2002.2323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schärer L., Vizoso D. B.2007Phenotypic plasticity in sperm production rate: there's more to it than testis size. Evol. Ecol. 21, 295–306 (doi:10.1007/s10682-006-9101-4) [Google Scholar]
- Schärer L., Joss G., Sandner P.2004Mating behaviour of the marine turbellarian Macrostomum sp.: these worms suck. Mar. Biol. 145, 373–380 [Google Scholar]
- Schärer L., Sandner P., Michiels N. K.2005Trade-off between male and female allocation in the simultaneously hermaphroditic flatworm Macrostomum sp. J. Evol. Biol. 18, 396–404 (doi:10.1111/j.1420-9101.2004.00827.x) [DOI] [PubMed] [Google Scholar]
- Snyder B. F., Gowaty P. A.2007A reappraisal of Bateman's classic study of intrasexual selection. Evolution 61, 2457–2468 (doi:10.1111/j.1558-5646.2007.00212.x) [DOI] [PubMed] [Google Scholar]
- St Mary C. M.1994Sex allocation in a simultaneous hermaphrodite, the blue-banded goby (Lythrypnus dalli)—the effect of body size and behavioral gender and the consequences for reproduction. Behav. Ecol. 5, 304–313 [Google Scholar]
- Sutherland W. J.1985Chance can produce a sex difference in variance in mating success and explain Batemans data. Anim. Behav. 33, 1349–1352 (doi:10.1016/S0003-3472(85)80197-4) [Google Scholar]
- Thornhill R.1983Cryptic female choice and its implications in the scorpionfly Harpobittacus nigriceps. Am. Nat. 122, 765–788 (doi:10.1086/284170) [Google Scholar]
- Vizoso D. B., Schärer L.2007Resource-dependent sex allocation in a simultaneous hermaphrodite. J. Evol. Biol. 20, 1046–1055 (doi:10.1111/j.1420-9101.2007.01294.x) [DOI] [PubMed] [Google Scholar]
- Wedell N., Gage M. J. G., Parker G. A.2002Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 17, 313–320 (doi:10.1016/S0169-5347(02)02533-8) [Google Scholar]
- Yund P. O., Marcum Y., Stewart-Savage J.1997Life-history variation in a colonial ascidian: broad-sense heritabilities and tradeoffs in allocation to asexual growth and male and female reproduction. Biol. Bull. 192, 290–299 (doi:10.2307/1542722) [DOI] [PubMed] [Google Scholar]