Abstract
The diversity and composition of biological communities might often depend on colonization history because early colonists can exclude future colonists through a priority effect. These priority effects, which have been observed across a wide variety of ecosystems, often arise because early colonists have sufficient time to use available resources efficiently and subsequently withhold them from invaders. Here, we explore the extent to which rapid local adaptive evolution contributes to the pervasiveness of these priority effects. Using an individual-based simulation, we show that early colonization allows the descendants of colonists to adapt to novel conditions and reduce the establishment success of an initially ecologically equivalent competing species. Our model predicts that slight differences in colonization timing and adaptive capacity between species can substantially alter the dynamics and diversity of communities. We also show that priority effects and gene flow can generate a novel mechanism for the expansion and retraction of species distributions in a metacommunity. Our results suggest that local adaptation combined with stochastic colonization events can obscure direct relationships between species distributions and environmental gradients. Given the increasing recognition of rapid, microgeographic evolution in natural populations, we expect that evolutionary priority effects could affect the structure and dynamics of many natural metacommunities.
Keywords: evolutionary ecology, gene flow, community coexistence, metapopulation, niche, community assembly
1. Introduction
Natural communities are dynamic in space and time, and understanding these joint dynamics represents one of ecology's greatest challenges. Ecologists have long recognized that colonization order can determine community structure through a priority effect, whereby early colonization affords a species an advantage in its interactions with future colonists (Sutherland 1974; Connell & Slatyer 1977; Shulman et al. 1983). Priority effects often arise because a species arrives early enough to increase in numbers, monopolize available resources and thereby gain a competitive numerical advantage over late-arriving colonists (Shulman et al. 1983). In this way, competitive priority effects are determined by the time it takes the initial species to colonize a patch and monopolize patch resources relative to the time it takes other competitors to colonize. Priority effects have been shown to affect community richness across a wide range of taxa and ecosystems (Connell & Slatyer 1977; Shulman et al. 1983; Wilbur & Fauth 1990; Shorrocks & Bingley 1994), alter the association between environmental conditions and species composition (Sutherland 1974), and increase the local prevalence of taxa with high dispersal capacities or high regional abundances (Holt & Hoopes 2005).
Although usually attributed to purely ecological mechanisms, priority effects also might be enhanced when genetic adaptation increases a resident population's performance in the local habitat. Past studies focusing on adaptive radiation on isolated islands have predicted that niche pre-emption will depend on the rates of speciation or niche expansion of early colonizing species relative to the immigration rates of better adapted species (Roughgarden 1972; Ricklefs & Bermingham 2002; Emerson & Gillespie 2008). Because speciation and niche evolution are generally expected to be slow processes, these ideas were developed to explain biogeographical patterns across macroevolutionary time-scales. However, similar processes might affect the dynamics of local communities given the growing recognition that populations can adapt over short time-scales (Kinnison & Hendry 2001; Hairston et al. 2005) and limited distances (Hendry & Taylor 2004; Nosil & Crespi 2004; Urban 2007).
The monopolization hypothesis, first developed to explain evolutionary priority effects in a single-species context (De Meester et al. 2002), can be expanded to explain how evolutionary priority effects affect community assembly (De Meester et al. 2007; Loeuille & Leibold 2008; Urban et al. 2008). Here, we define dispersal as the displacement of an individual from its birth place such that the individual immigrates into a non-natal patch. We refer to colonization as the immigration of a new species to a patch. Immigration does not necessarily guarantee the migrant's survival and successful reproduction. We therefore define establishment as the arrival, survival and subsequent contribution of offspring to the reproductive pool by an immigrant, a definition consistent with that describing gene flow (Whitlock & McCauley 1999), in order to differentiate it from immigration, by which we mean arrival only. The monopolization hypothesis suggests that immigration often will not equate with establishment and subsequent gene flow because locally adapted residents prevent late immigrants from contributing to the gene pool (De Meester et al. 2002). In particular, we expect that a resident population will reduce an immigrant's ability to reproduce whenever residents can monopolize resources or selection acts against maladapted immigrants (De Meester et al. 2002; Hendry & Taylor 2004; Nosil et al. 2005). As a result, even though a good disperser arrives in many habitats, these immigrants will not always successfully establish and reproduce in those habitats. As such, even good dispersers can become locally adapted, a pattern long recognized but not often explained (Ehrlich & Raven 1969; Hendry & Taylor 2004). In the context of a community of competitors, we expect that rapid adaptation in early-arriving species will prevent late-arriving species from establishing in a community just as rapid adaptation can prevent the establishment of invading genotypes at the population level.
We developed an individual-based model of a two-species, three-patch evolving metacommunity with stochastic dispersal and mutation. In contrast to prior work (Roughgarden 1972; Loeuille & Leibold 2008), we explore how differences in the dispersal abilities and adaptabilities among competing, initially ecologically neutral species interact to determine community assembly in a stable habitat. We first manipulate the initial colonization times of competing species in the new environment, while maintaining equivalent dispersal rates, to tease apart the individual contribution of early colonization and adaptation on community assembly in the absence of the confounding effects of correlated dispersal and gene flow-induced maladaptation. Besides establishing the importance of the history of colonization in determining future competitive dynamics, this scenario also corresponds to one in which species colonize patches distant from source habitats, such that stochastic long-distant dispersal events occur (e.g. oceanic islands or human-facilitated introductions). We then let differences in dispersal ability among species directly determine colonization timing and explore how the combined effects of immigration, gene flow and local adaptation affect community dynamics. Across a range of assumptions about dispersal and evolutionary rates, we demonstrate that local adaptation often allows an early colonist to dominate a habitat patch.
2. Material and Methods
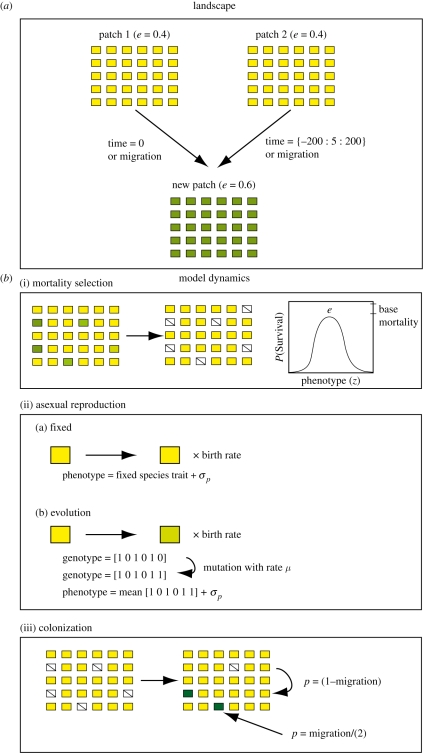
We assume two patches with the same environment (e = 0.4) and an initially vacant habitat with a different fixed environment (e = 0.6) (figure 1). We assume a lottery competition for 1000 available microsites in each patch. Microsites vary according to a Gaussian function of the mean patch environment and environmental breadth σe2. As in other lottery models, recruitment probability depends on the prevalence of each species and genotype in the regional reproductive pool.
Figure 1.
We developed an individual-based model of community assembly in a three-patch, two-species metacommunity. (a) Two initially equivalent species with traits matching the environment fill two patches, each with 1000 microsites and an environmental value (e) of 0.4 (symbolized in yellow). A third vacant patch with a different environment (e = 0.6, symbolized in green) is colonized by either the first or second species, followed by the other species with some time lag from 0 to 200 time steps or depending on dispersal probability. (b) Population dynamics are determined by (i) mortality selection, (ii) reproduction, and (iii) immigration into empty microsites by offspring. During mortality selection, a survival probability is calculated for each individual in each microsite as a Gaussian function of the individual's phenotype (z) and the microsite environment (e) minus a fixed base mortality decrement. Adults die based on a stochastic realization of this survival probability. Surviving adults reproduce asexually with or without evolution by mutation or standing genetic variation. A small random variance is added to each phenotype to represent non-genetic random contributions to the phenotype. The number of offspring per adult per time step was determined by a fixed birth rate (r). In evolutionary simulations, an individual's phenotype was determined as the mean of a bi-allelic string of ones and zeros. Mutation occurs with rate μ and can generate a new phenotype (indicated in green). In each patch, a total immigrant pool is calculated as the sum of all individuals in the metacommunity weighted by their immigration probability into that patch and multiplied by birth rate. All empty microsites are filled up to the total number of immigrants by a process of random sampling with replacement, weighted by the probability of staying (from the same patch) or immigrating (from the other two patches). The process then is repeated for 5000 time steps.
Initially, two haploid asexual species with discrete reproduction and overlapping generations colonize the empty novel environment after differing time periods (first set of simulations) or as a function of their respective dispersal probabilities (second set). The base model begins with two species that are initially ecologically and evolutionarily equivalent in all characteristics. However, in models allowing evolution, the two species can diverge in a niche-related trait that determines their survival in different environments. Hence, our model does not include any fixed niche differences at the outset, but these niche differences can evolve. Additional models explore how non-neutral differences among species in dispersal ability or adaptive potential alter these results. At the start of a simulation, each species has a phenotype that matches its source environment plus a random normal variable (mean = 0, variance = σp2) reflecting non-genetic contributions (figure 1). In cases including evolution, one or both species evolve 20 bi-allelic, additive genes coded as ones or zeros that are averaged to determine their phenotype (e.g. a genotype with 10 ones and 10 zeros generates a phenotype of 0.5). We explore two major routes through which genetic variation emerges: mutation and standing allelic variation. A mutation occurs in the model when mutation rate (μ) exceeds a uniform random variable assigned to each allele. Initial standing genetic variation is modelled by randomly assigning ones and zeros to alleles with a probability equal to the mean phenotype.
At each time step, the survival of each individual i (Sij) in microsite j is a Gaussian function of their phenotype (zi), and the microsite environment (ej):
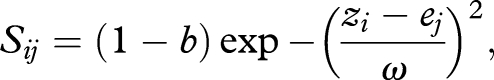 |
2.1 |
where b is a baseline mortality rate and ω indicates the width in standard deviations of stabilizing selection. An individual dies if Sij is less than a uniform random variable [0,1]. Following mortality selection and offspring production, individuals migrate into empty microsites by random sampling with replacement. The probability of migrating into an empty microsite equals one minus emigration probability for residents and one-half the emigration probability for immigrants. The total number of immigrants to patch j (Ij) equals
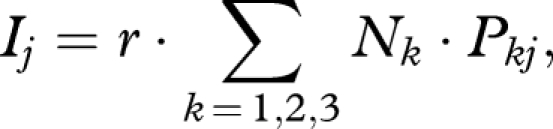 |
2.2 |
the total number of individuals in each patch (Nk) multiplied by their offspring's probabilities of staying in the patch (k=j) or immigrating to patch j from other patches (Pkj) times the birth rate (r), assumed to be the same for each species and genotype.
In the first scenario, we allow species to colonize the new patch in five time-step increments from an advantage in the colonization time of 200 time steps for one species to an advantage of 200 time steps for the other. In the second scenario, we evaluated outcomes for all combinations of 11 dispersal probabilities ranging from 0.005 to 0.5. Each simulation ran for 5000 time steps. The results of simulations lasting 20 000 times steps did not differ qualitatively from those run for 5000 time steps (electronic supplementary material, figure S1). We recorded the mean abundances of both species after 100 simulations for each set of parameters explored and across the full range of colonization times (electronic supplementary material, table S1).
3. Model results
(a). Monopolization effects depend on colonization time and evolutionary potential
Our first objective is to demonstrate the potential for adaptive priority effects when a species arrives first without the confounding effects of dispersal-associated differences in gene flow. We therefore initially explore how differences in the evolutionary potential and colonization time jointly determine the abundances of two competing species while keeping interpatch dispersal probability fixed. This theoretical framework simulates a classic experimental demonstration of ecological priority effects in fruitflies (Shorrocks & Bingley 1994) but over multiple generations of evolution. This setup could represent a scenario in which one species colonizes a new habitat by chance or through long-distance transport. In §3b, dispersal ability directly determines colonization time.
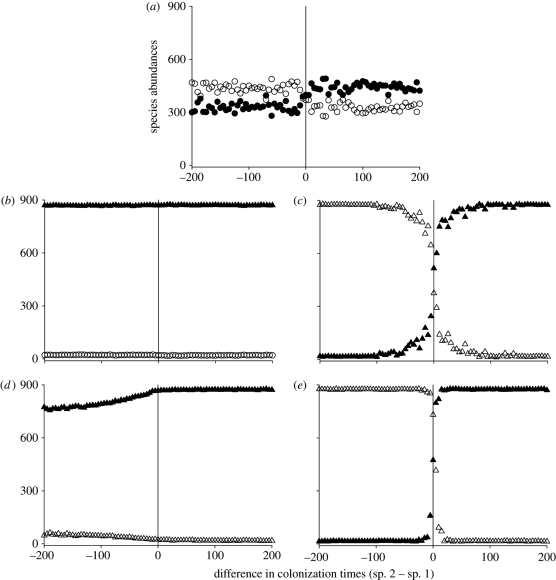
Without evolution, ecological priority effects usually allow the first species to dominate the community (figure 2a). However, this effect is weak, and the identity of the dominant species varies considerably from run to run because neutral ecological drift sometimes allows the early-arriving species to go extinct and the late-arriving species to dominate.
Figure 2.
The mean final abundances (n = 100 simulations) of two initially neutrally competitive species differ depending on their colonization times and adaptive potentials. In each subpanel, the bottom axis describes the difference in times of introduction between the two species. At the central vertical line, both species colonize the new habitat at the same time. To the left of this line, species 2 was introduced before species 1 (negative values). To the right of this line, species 1 was introduced before species 2 (positive values). (a) Neither species evolves. Each species dominates when they colonize the habitat before the other, but this priority effect is not very strong. Black circles, species 1; white circles, species 2. (b,c) Evolution occurs through mutation and (d,e) through standing genetic variation. (b,d) Species 1 (black symbols) evolves, whereas species 2 (white) does not. The evolving species dominates across the range of colonization times. (c,e) When both species evolve, each species dominates the patch when introduced earlier than the other, and this dominance is strongly augmented by evolution-mediated priority effects. Black triangles, evolving species 1; white triangles, evolving species 2. Parameter values follow those indicated as baseline in electronic supplementary material, table S1.
Evolution considerably alters competitive outcomes. When one species evolves and the other does not, the evolving species dominates the patch over the non-evolving species across all colonization times (figure 2b,d) even though the two species initially had equal fitness in the new patch. The evolving species dominates a community even when introduced late because the non-evolving resident has low fitness in the new patch and thus never fully monopolizes all resources. Resource availability allows the evolving species to persist in the new habitat while favourable mutations arise and facilitate its higher relative fitness and eventual monopolization of the community. In these simulations, differences in adaptability override numerical priority effects. In the case of standing genetic variation, pre-existing genotypes with higher fitness in the new habitat arrive and come to dominate it (figure 2d). The species that evolves via standing genetic variation instead of mutation dominates the community less when introduced later than the first species (cf. figure 2b,d) because natural selection and genetic drift erode standing genetic variation in the source population before its individuals can colonize the new habitat.
When both species can evolve, the species that arrives first dominates the community, supporting the main prediction of the community monopolization hypothesis that adaptation combined with early colonization should lead to community dominance (figure 2c,e). This monopolization effect is powerful and occurs even if a species only arrives five to 10 time steps before the other. The effect arises when one considers evolution by mutations or standing genetic variation. A stronger monopolization effect occurs for simulations with standing genetic variation compared with mutational variation because sufficient genetic variation immediately exists to fuel an evolutionary response after colonization (figure 2e).
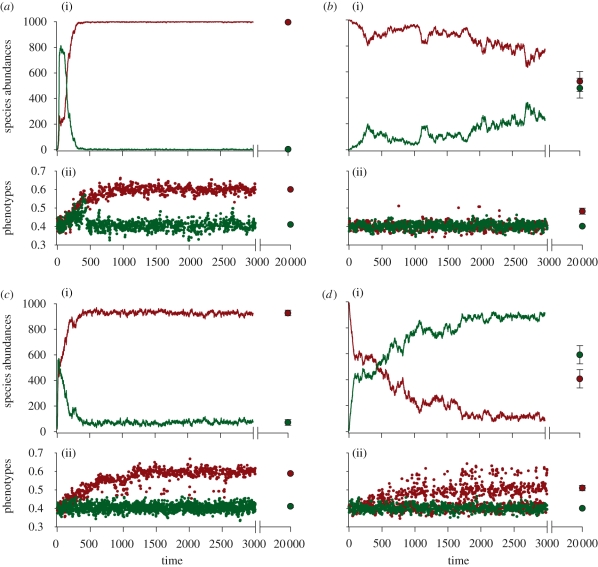
We also explore simulations in which the interpatch dispersal probabilities were lower (m = 0.005) and higher (m = 0.10) than in the baseline model (m = 0.05). Lower dispersal increases the monopolization effect (electronic supplementary material, figure S2). Variation in dispersal rates also substantially alters the distribution of species among source patches. Low dispersal (m < 0.05) allows species 1, which dominates the new patch, to adapt to both original and new habitats, whereas species 2 only dominates its original source habitat (figure 3a,b). In the original patch of species 1, species 1 retains a transient advantage over species 2, which eventually equilibrates after 20 000 time steps owing to maladaptive gene flow from the new patch (figure 3b). Under higher dispersal (m = 0.10), species 1 adapts to the new patch, but species 2 rapidly replaces it in its source patch (figure 3c,d). This dominance by the second species remains after 20 000 time steps. Higher gene flow prevents species 1 from adapting to both environments and, as a result, it becomes maladapted to its original source habitat (figure 3d(ii)). Species 2 does not suffer from this genetic load and thus can replace species 1 in its source patch.
Figure 3.
A species that monopolizes a new patch through adaptation can become a victim of its own success when dispersal is high. We assume two evolving species with similar adaptabilities. At low dispersal (m = 0.01), two evolving species inhabiting two similar environments (e = 0.4) colonize a new environment (e = 0.6) at the same time. We depict the species abundances and phenotypes of both species in the new patch (first column) and in the original patch of species 1 (second column). Filled circles indicate the mean abundances and phenotypic values (±1 s.e.) for 40 simulations after 20 000 time steps. (a(i)(ii)) By chance, species 1 (red) adapts faster than species 2 (green) and comes to dominate the new patch. (b(i)(ii)) Species 1 remains adapted to its original source patch for a transitory period until its abundance equilibrates with that of species 2. (c(i)(ii)) Species 1 again wins the new patch by chance, adapts to the new conditions and dominates the patch. However, higher dispersal (m = 0.10) increases the maladaptive gene flow from the new habitat (c(i)(ii)) to the original source habitat of species 1. As a consequence, the original source population of species 1 becomes increasingly maladapted (d(i)(ii)) and declines in abundance substantially relative to species 2. This effect remains after 20 000 time steps. For results at time = 20 000, the symbol frequently hides the small error bars.
In addition, we examine how different mutation rates affect monopolization patterns by altering adaptive potential. Monopolization effects still occur at lower mutation rates (μ = 1 × 10−6) but are weaker owing to decreased evolutionary potential (electronic supplementary material, figure S3). When species differ in their mutation rates, but not dispersal abilities, the species that can adapt faster generally dominates the new community (electronic supplementary material, figure S4).
(b). Monopolization effects when colonization timing depends on dispersal
We next evaluate monopolization effects under the more complex and common situation whereby dispersal ability directly determines each species' colonization time, rather than manipulating colonization time independent of subsequent dispersal probabilities. These simulations allow gene flow to swamp local adaptation in the better dispersing species and introduce more colonization stochasticity because poor dispersers sometimes colonize a patch by chance. If monopolization effects still occur under these conditions, then each species should dominate the new patch when it disperses better than the other species.
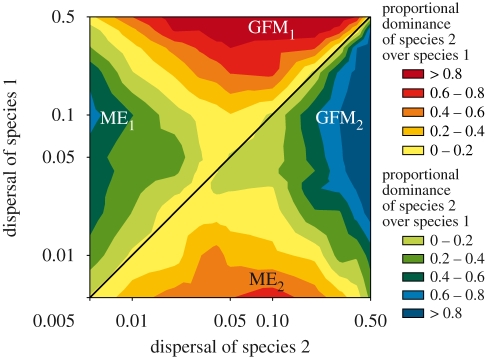
When dispersal directly determines colonization timing, we again find that community monopolization effects occur when a species disperses better than its competitor (figure 4, two regions indicated as ME). However, in this case, monopolization effects do not occur when a species disperses so much that gene flow-induced maladaptation overrides the evolutionary advantage of early colonization (figure 4, indicated by GFM). At these higher dispersal probabilities, gene flow limits the ability of the best disperser to adapt to the new habitat, which allows the poor disperser to monopolize the patch through adaptation. Maladaptation was the most important dynamic in the parameter space explored (note the logarithmic scale of dispersal in figure 4, which under-emphasizes the area of maladaptation). Monopolization effects should occur whenever competing species differ in their dispersal capabilities and the best disperser does not exceed the dispersal probability that swamps its local adaptation. As the threshold for gene flow-induced maladaptation will depend on the strength of antagonistic selection between habitats (Slatkin 1987), monopolization effects should occur more frequently in metacommunities with highly divergent local selection regimes or with patches separated by long distances.
Figure 4.
Monopolization effects occur when colonization timing depends directly on interpatch dispersal probability. Here we explored all combinations of 11 interpatch dispersal probabilities among two evolving species, ranging from 0.005 to 0.5. Note the logarithmic scale, which emphasizes results at lower dispersal probabilities. Outcomes are interpolated across these 121 results. Colours indicate the relative proportional dominance (difference in abundance between the two species in the new patch divided by total possible abundance) of one species over the other in relation to their respective dispersal abilities on a log–log dispersal plot. The central diagonal line indicates where species' dispersal rates equal each other. Above and to the left of this line, species 1 disperses better than species 2; below this line, species 2 disperses better. Monopolization effects occur when a species disperses better than its competitor (indicated by ME with a subscript that indicates the species to which it applies) as long as its dispersal probability is not so high that gene flow-induced maladaptation in the better disperser (indicated by GFM) gives the poorer disperser the evolutionary advantage in the new patch. The region of maladaptation is determined by the swamping effect of gene flow relative to the advantage provided by the monopolization effect.
4. Discussion
Ecological priority effects have been shown to occur in many natural communities (Connell & Slatyer 1977; Shulman et al. 1983; Wilbur & Fauth 1990; Gillespie 2004) and to affect community assembly experimentally (Drake 1991; Shorrocks & Bingley 1994) and theoretically (MacArthur 1970; Levins & Culver 1971). However, our current understanding of priority effects neglects the effect of rapid, localized adaptation on communities. Our simulations demonstrate that evolution-mediated priority effects can profoundly shape community assembly. Our results highlight the importance of historical colonization events, local adaptation and contemporary gene flow in determining patterns of community differentiation and diversity.
(a). Model conclusions and predictions
We find support for community monopolization effects in our simulations across a range of dispersal rates and adaptive potentials. Early arrival supplies colonists with enough time to adapt to local conditions and to decrease invasions by subsequent colonists in our simulations (figures 1 and 3). Adaptation was facilitated by the persistence of a small population in the new habitat following colonization, which allowed favourable mutations to arise. This initial adaptation increased population size, leading to a rapid accumulation of additional favourable mutations (Holt et al. 2003). Thus, the monopolization effect can be a self-reinforcing process (sensu De Meester et al. 2002), which explains why a small difference in colonization times can result in two radically different community compositions. In the initial set of simulations, we assumed that one species colonized before the other even though they did not differ in dispersal rates thereafter. This setup allowed us to explore the direct role that early arrival plays in determining ecological and evolutionary dynamics. This scenario corresponds to one in which a set of patches was colonized far from source patches as might be the case among widely separated patches (e.g. islands) or serial long-distance introductions of species by humans. In these cases, we expect that early introductions will exclude later introductions if early colonists have sufficient time to adapt to these conditions. At least in some cases, evidence suggests that early introductions can exclude later introductions through competition (Simberloff & Von Holle 1999). However, the role of evolution in affecting the success of serial invasions has yet to be evaluated.
The overall predictions of our first model were robust to the joint effects of dispersal and gene flow at low-to-moderate dispersal probabilities (figure 4). Under covarying dispersal and colonization rates, community monopolization effects depend both on the relative difference between dispersal abilities among species and the relationship between the gene flow in the best disperser relative to differences in selection pressures among habitats (environmental heterogeneity). We can expect traditional gene flow-induced maladaptation when dispersal exceeds selection strength (Mayr 1963; Slatkin 1987). However, the range of dispersal rates supporting monopolization effects falls within the range commonly observed for natural populations. For example, Morjan & Rieseberg (2004) found that most populations were linked by four or less migrants per generation, which would support an average migration rate of less than 4 per cent, assuming a moderate effective population size of 100. We therefore suggest that monopolization could occur commonly in natural communities.
Our results suggest a greater role for monopolization effects in shaping community dynamics as compared with the only other model to evaluate these effects explicitly (Loeuille & Leibold 2008). This previous model differed from ours by assuming a temporally varying selection environment and initial niche differences among non-evolving competitors. The dynamic environment assumed in this model usually prevented monopolization effects by allowing niche-differentiated non-evolving species to supplant evolving species which had phenotypes that lagged behind the changing environment (Loeuille & Leibold 2008). Taking these and our results together, we can expect monopolization effects when competing species differ in dispersal ability and environments remain similar over the time-scales during which adaptation occurs. In many natural systems, empirical evidence indicates that major selection gradients (e.g. predator presence, soil chemistry) remain constant enough over time that local populations have sufficient time to adapt to them (Cousyn et al. 2001; Hendry & Taylor 2004; Urban 2007). However, direct empirical tests will be needed to demonstrate the overall importance of monopolization effects in natural systems and to verify the conditions expected to generate them.
Our simulations also show that species with more genetic variation should dominate local communities (figure 2: compare (b) or (d) versus (a)). This prediction gains support from research on invasive species, where an introduced population's derivation from multiple source populations enhances its genetic variation, which, in turn, aids its successful adaptation and spread in exotic habitats (Kolbe et al. 2004; Lavergne & Molofsky 2007). One could also test this prediction experimentally by evaluating the outcome of interactions between species that differ in the heritabilities of key traits in natural or experimental metacommunities.
Different dispersal probabilities only weakly affected the strength of the monopolization effect when both species evolve (electronic supplementary material, figure S2). However, differences in dispersal substantially affected the distribution of species in the metacommunity. The observed dynamics suggest a potentially important mechanism of expansion and retraction of species distributions in a metacommunity. In this mechanism, a competitor first adapts to the new patch and excludes the other species. The newly adapted population then sends maladapted genes back to its source patch, which lowers its overall fitness there relative to the other species (figure 3). The second species gains a fitness advantage in the newly adapted species' original patch owing to the other species' maladaptation there and can thereby dominate both original sources patches. Thus, the winner of the new patch becomes the victim of its own success and realizes a changed, but not expanded, distribution when dispersal is high. Under low dispersal, a species remains adapted to both its source habitat and the new habitat because gene flow does not swamp local adaptation. Loeuille & Leibold (2008) also showed that an evolving species which adapts to one environment fails to adapt to a temporally variable environment owing to gene flow. These results suggest a general evolution-mediated mechanism of sink-source reversals that differs from those expected to occur through disturbance (Ronce & Kirkpatrick 2001; Holt et al. 2005).
The occurrence of monopolization effects depends on differences in dispersal ability and adaptability among regional species, how closely these species compete for similar niches and their relative rates of competitive exclusion versus evolutionary divergence. Adaptive priority effects could also occur in communities with weak competitive interactions if early arrival and adaptation alter species interaction types other than competition (Meyer & Kassen 2007). Niche differences among competitors will further determine adaptive priority effects. For instance, the existence of a better adapted competitor in the regional species pool will lessen the probability that an initially less-suited species will have sufficient time to adapt to a new habitat before the better suited species arrives (Loeuille & Leibold 2008). Our model began with initially equivalent species. Evaluating how initial niche differences among species affect model results provides an interesting future direction for this research. As rapid local adaptation fundamentally determines monopolization effects, we expect them to be more pronounced in species with short generation times and substantial genetic variation for ecologically relevant traits. The need for high genetic variability to dominate a patch also emphasizes the importance of the reproductive mode (asexual versus sexual), but this prediction needs to be explored further both theoretically and experimentally.
(b). Ecological and evolutionary history
Our results suggest that random historical events during community assembly and evolution can lead to alternative community states. In simulations involving two evolving species across a range of parameter values, we observe a U-shaped frequency distribution of each species' abundances, with a species reaching either very high or very low densities in the new patch. A combination of early colonization and evolution-mediated priority effects strengthens this pattern. This pattern occurs, in part, because monopolization effects can reinforce initial asymmetries in population abundances (see also Scheffer & Van Nes 2006). Monopolization by local adaptation becomes a self-reinforcing process, as occurred in our model of competition for space, when a favourable mutation arises in a population that supports larger population sizes, greater monopolization of resources and a higher probability that additional favourable mutations will occur. Monopolization effects might occur less often in situations in which traits evolve that increase individual fitness but decrease population size. However, for the common situation in which individual fitness gains also lead to larger population sizes, monopolization effects can allow for a given environment to harbour completely different communities owing to the reinforcing effects of adaptive evolution (figures 2 and 4).
Sometimes in our simulations, a species dominated a community even when it did not arrive first owing to stochastic demographic and evolutionary events. Our simulations began with neutral species that then diverged in a niche-related trait through local adaptation. Stochasticity in colonization times or evolutionary trajectories can introduce noise into the match between community composition and environmental conditions, generating patterns superficially similar to those occurring through neutral dynamics. These patterns would, however, not reflect the neutral dynamics of ecologically equivalent species, but rather the importance of random colonization events and subsequent adaptation to local conditions (Scheffer & Van Nes 2006). These evolutionary niche dynamics could explain why more apparently neutral patterns are observed in nature than expected based on the ecologies of constituent species (e.g. Cottenie 2005; Holyoak & Loreau 2006). The community monopolization hypothesis offers an explanation for why different communities develop in ecologically equivalent habitats in the absence of strong barriers to dispersal. The key mechanism is that adaptation can strongly enhance priority effects under a broad range of conditions.
(c). Empirical evidence
Empirical evidence supports the basic assumption of the community monopolization hypothesis, that the genotype of an initial colonist can alter subsequent community assembly. In experimental pond communities, Daphnia magna water fleas that were adapted to different source habitats altered the establishment success of other zooplankton species from the regional species pool (De Meester et al. 2007). In old fields, Crutsinger et al. (2008) showed that the genotypic identity of the goldenrod Solidago altissima influenced the establishment success of colonizing species. In Populus trees, individual host tree genotype determines arthropod community structure (Whitham et al. 2006). These examples indicate that the genotype of a focal species can determine community assembly. However, none of these studies explicitly tests the central prediction that early colonization allows for local adaptation to affect community assembly. In one exemplary experimental study, the time at which different bacteria (Pseudomonas fluorescens) ecomorphs were introduced into an experimental community was shown to affect future patterns of niche diversification among the bacteria (Fukami et al. 2007). At macroevolutionary time-scales, the phylogenies and traits of Hawaiian orbweaver spiders (Tetragnatha) demonstrate that long intervals between colonization events provided the time needed for residents to radiate into different ecomorphs to fill available niches on some islands, whereas on other islands, pre-adapted immigrants arrived to fill those niches before residents could speciate (Gillespie 2004). Additional future experiments should be designed to manipulate colonization times and adaptabilities among competing species to test the predictions generated by our simulations.
5. Conclusions
Community ecologists have long recognized that early colonization by one species can decrease the invasion success of subsequent colonists (Levins & Culver 1971; Connell & Slatyer 1977; Shulman et al. 1983). Yet, the same time lag between colonization events also provides the opportunity for adaptive evolution to enhance priority effects (Roughgarden 1972; De Meester et al. 2007; Fukami et al. 2007; Loeuille & Leibold 2008). Evolutionary and ecological dynamics can occur on similar time-scales (Kinnison & Hendry 2001; Hairston et al. 2005), implying that evolution might sometimes alter ecological dynamics (e.g. Thompson 2005; Urban et al. 2008). The community monopolization hypothesis predicts that local genetic adaptation affects community assembly. Under this hypothesis, the diversity and composition of a community depend on a race between local adaptation of residents and colonization and adaptation of other species. Overall, model predictions and empirical support for effects of adaptations on community assembly suggest that community monopolization effects might occur frequently in nature, thereby providing a possible explanation for why priority effects occur so frequently in natural communities. We therefore caution ecologists not to always attribute priority effects to ecological mechanisms alone—evolution might frequently play a role.
Acknowledgements
This work was conducted as part of the Evolving Metacommunity Working Group supported by the National Center for Ecological Analysis and Synthesis (NCEAS). Additional support was provided to M.C.U. as an NCEAS Postdoctoral Associate and to L.D.M. by KULeuven Research Fund project GOA/2008/06. We thank M. Leibold, N. Loeuille, A. Magurran, F. Van de Meutter and an anonymous reviewer for their comments on earlier drafts.
References
- Connell J. H., Slatyer R. O.1977Mechanisms of succession in natural communities and their role in community stability and organization. Am. Nat. 111, 1119–1144 (doi:10.1086/283241) [Google Scholar]
- Cottenie K.2005Integrating environmental and spatial processes in ecological community dynamics. Ecol. Lett. 8, 1175–1182 (doi:10.1111/j.1461-0248.2005.00820.x) [DOI] [PubMed] [Google Scholar]
- Cousyn C., De Meester L., Colbourne J. K., Brendonck L., Verschuren D., Volckaert F.2001Rapid, local adaptation of zooplankton behavior to changes in predation pressure in the absence of neutral genetic changes. Proc. Natl Acad. Sci. USA 98, 6256–6260 (doi:10.1073/pnas.111606798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutsinger G. M., Souza L., Sanders N. J.2008Intraspecific diversity and dominant genotypes resist plant invasions. Ecol. Lett. 11, 16–23 [DOI] [PubMed] [Google Scholar]
- De Meester L., Gomez A., Okamura B., Schwenk K.2002The monopolization hypothesis and the dispersal-gene flow paradox in aquatic organisms. Acta Oecol. 23, 121–135 (doi:10.1016/S1146-609X(02)01145-1) [Google Scholar]
- De Meester L., Louette G., Duvivier C., Van Damme C., Michels E.2007Genetic composition of resident populations influences establishment success of immigrant species. Oecologia 153, 431–440 [DOI] [PubMed] [Google Scholar]
- Drake J. A.1991Community-assembly mechanics and the structure of an experimental species ensemble. Am. Nat. 137, 1–26 (doi:10.1086/285143) [Google Scholar]
- Ehrlich P. R., Raven P. H.1969Differentiation of populations. Science 165, 1228–1232 (doi:10.1126/science.165.3899.1228) [DOI] [PubMed] [Google Scholar]
- Emerson B. C., Gillespie R. G.2008Phylogenetic analysis of community assembly and structure over space and time. Trends Ecol. Evol. 23, 619–630 (doi:10.1016/j.tree.2008.07.005) [DOI] [PubMed] [Google Scholar]
- Fukami T., Beaumont H. J. E., Zhang X.-X., Rainey P. B.2007Immigration history controls diversification in experimental adaptive radiation. Nature 446, 436–439 (doi:10.1038/nature05629) [DOI] [PubMed] [Google Scholar]
- Gillespie R.2004Community assembly through adaptive radiation in Hawaiian spiders. Science 303, 356–359 (doi:10.1126/science.1091875) [DOI] [PubMed] [Google Scholar]
- Hairston N. G., Ellner S. P., Geber M. A., Yoshida T., Fox J. A.2005Rapid evolution and the convergence of ecological and evolutionary time. Ecol. Lett. 8, 1114–1127 (doi:10.1111/j.1461-0248.2005.00812.x) [Google Scholar]
- Hendry A. P., Taylor E. B.2004How much of the variation in adaptive divergence can be explained by gene flow? An evaluation using lake–stream stickleback pairs. Evolution 58, 2319–2331 [DOI] [PubMed] [Google Scholar]
- Holt R. D., Hoopes M.2005Food web dynamics in a metacommunity context: modules and beyond. In Metacommunities: spatial dynamics and ecological communities (eds Holyoak M., Leibold M. A., Holt R. D.), pp. 68–93 Chicago, IL: University of Chicago Press [Google Scholar]
- Holt R. D., Gomulkiewicz R., Barfield M.2003The phenomenology of niche evolution via quantitative traits in a ‘black-hole’ sink. Proc. R. Soc. Lond. B 270, 215–224 (doi:10.1098/rspb.2002.2219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt R. D., Barfield M., Gomulkiewicz R.2005Theories of niche conservatism and evolution. In Species invasions: insights into ecology, evolution, and biogeography (eds Sax D. F., Stachowicz J. J., Gaines S. D.), pp. 259–290 Sunderland, MA: Sinauer Associates, Inc [Google Scholar]
- Holyoak M., Loreau M.2006Reconciling empirical ecology with neutral community models. Ecology 87, 1370–1377 (doi:10.1890/0012-9658(2006)87[1370:REEWNC]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Kinnison M. T., Hendry A. P.2001The pace of modern life. II: from rates of contemporary microevolution to pattern and process. Genetica 112–113, 145–164 (doi:10.1023/A:1013375419520) [PubMed] [Google Scholar]
- Kolbe J. J., Glor R. E., Schettino L. R., Lara A. C., Larson A., Losos J. B.2004Genetic variation increases during biological invasion by a Cuban lizard. Nature 431, 177–181 (doi:10.1038/nature02807) [DOI] [PubMed] [Google Scholar]
- Lavergne S., Molofsky J.2007Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proc. Natl Acad. Sci. USA 104, 3883–3888 (doi:10.1073/pnas.0607324104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levins R., Culver D.1971Regional coexistence of species and competition between rare species. Proc. Natl Acad. Sci. USA 68, 1246–1248 (doi:10.1073/pnas.68.6.1246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeuille N., Leibold M. A.2008Evolution in metacommunities: on the relative importance of species sorting and monopolization in structuring communities. Am. Nat. 171, 788–799 (doi:10.1086/587745) [DOI] [PubMed] [Google Scholar]
- MacArthur R. H.1970Species-packing and competitive equilibrium for many species. Theor. Popul. Biol. 1, 1–11 (doi:10.1016/0040-5809(70)90039-0) [DOI] [PubMed] [Google Scholar]
- Mayr E.1963Animal species and evolution Cambridge, MA: Harvard University Press [Google Scholar]
- Meyer J. R., Kassen R.2007The effects of competition and predation on diversification in a model of adaptive radiation. Nature 446, 432–435 (doi:10.1038/nature05599) [DOI] [PubMed] [Google Scholar]
- Morjan C. L., Rieseberg L. H.2004How species evolve collectively: implications of gene flow and selection for the spread of advantageous alleles. Mol. Ecol. 13, 1341–1356 (doi:10.1111/j.1365-294X.2004.02164.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosil P., Crespi B. J.2004Does gene flow constrain adaptive divergence or vice versa? A test using ecomorphology and sexual isolation in Timema cristinae walking-sticks. Evolution 58, 102–112 [DOI] [PubMed] [Google Scholar]
- Nosil P., Vines T. H., Funk D. J.2005Reproductive isolation caused by natural selection against immigrants from divergent habitats. Evolution 59, 705–719 [PubMed] [Google Scholar]
- Ricklefs R. E., Bermingham E.2002The concept of the taxon cycle in biogeography. Global Ecol. Biogeogr. 11, 353–361 (doi:10.1046/j.1466-822x.2002.00300.x) [Google Scholar]
- Ronce O., Kirkpatrick M.2001When sources become sinks: migrational meltdown in heterogeneous habitats. Evolution 55, 1520–1531 [DOI] [PubMed] [Google Scholar]
- Roughgarden J.1972Evolution of niche width. Am. Nat. 106, 683–718 (doi:10.1086/282807) [Google Scholar]
- Scheffer M., Van Nes E. H.2006Self-organized similarity, the evolutionary emergence of groups of similar species. Proc. Natl Acad. Sci. USA 103, 6230–6235 (doi:10.1073/pnas.0508024103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorrocks B., Bingley M.1994Priority effects and species coexistence: experiments with fungal-breeding Drosophila. J. Anim. Ecol. 63, 799–806 (doi:10.2307/5257) [Google Scholar]
- Shulman M. J., Ogden J. C., Ebersole J. P., McFarland W. N., Miller S. L., Wolf N. G.1983Priority effects in the recruitment of juvenile coral fishes. Ecology 64, 1508–1513 (doi:10.2307/1937505) [Google Scholar]
- Simberloff D., Von Holle B.1999Positive interactions of nonindigenous species: invasional meltdown? Biol. Invasions 1, 21–32 (doi:10.1023/A:1010086329619) [Google Scholar]
- Slatkin M.1987Gene flow and the geographic structure of natural populations. Science 236, 787–792 (doi:10.1126/science.3576198) [DOI] [PubMed] [Google Scholar]
- Sutherland J. P.1974Multiple stable points in natural communities. Am. Nat. 108, 859–873 (doi:10.1086/282961) [Google Scholar]
- Thompson J. N.2005The geographic mosaic of coevolution Chicago, IL: University of Chicago Press [Google Scholar]
- Urban M. C.2007Risky prey behavior evolves in risky habitats. Proc. Natl Acad. Sci. USA 104, 14 377–14 382 (doi:10.1073/pnas.0704645104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban M. C., et al. 2008The evolutionary ecology of metacommunities. Trends Ecol. Evol. 23, 311–317 (doi:10.1016/j.tree.2008.02.007) [DOI] [PubMed] [Google Scholar]
- Whitham T. G., et al. 2006A framework for community and ecosystem genetics: from genes to ecosystems. Nat. Rev. Genet. 7, 510–523 (doi:10.1038/nrg1877) [DOI] [PubMed] [Google Scholar]
- Whitlock M. C., McCauley D. E.1999Indirect measures of gene flow and migration: FST≠1/(4Nm+1). Heredity 82, 117–125 (doi:10.1038/sj.hdy.6884960) [DOI] [PubMed] [Google Scholar]
- Wilbur H. M., Fauth J. E.1990Experimental aquatic food webs: interactions between two predators and two prey. Am. Nat. 135, 176–204 (doi:10.1086/285038) [Google Scholar]