Table 1.
Cross aldol reaction of ethyl (benzoylphenyl)phosphinate (5a) and acetone (6a) catalyzed by L-proline derivativesa
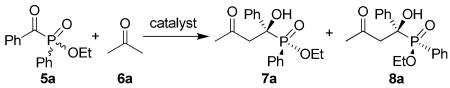 | |||||||
|---|---|---|---|---|---|---|---|
| entry | catalyst | loadingb | solvent | yield (%)c | ee (%)d | 7a/8ae | |
| 7a | 8a | ||||||
| 1f | 1 | 10 | acetone | 93 | 72 | 47 | 55:45 |
| 2f | 2 | 10 | acetone | 63 | 45 | 28 | 58:42 |
| 3f | 3 | 10 | acetone | 83 | 82 | 62 | 54:46 |
| 4f | 4 | 20 | acetone | 89 | 95 | 74 | 53:47 |
| 5 | 4 | 20 | THF | 90 | 95 | 67 | 52:48 |
| 6 | 4 | 20 | CH2Cl2 | 79 | 94 | 65 | 50:50 |
| 7 | 4 | 20 | DMSO | 81 | 52 | 32 | 48:52 |
| 8 | 4 | 20 | DMF | 81 | 69 | 39 | 58:42 |
| 9g | 4 | 20 | acetone | 87 | 91 | 59 | 59:41 |
| 10f,h | 4 | 20 | acetone | 58 | 99 | 91 | 52:48 |
Unless otherwise indicated, all reactions were carried out with the ketophosphinate 5a (0.50 mmol), acetone (0.5 mL), and the catalyst in the specified solvent (1.0 mL) at rt for 24 h.
mol %.
Total yield of the inseparable diastereomers (7a and 8a) isolated after column chromatography.
Determined by HPLC analyses.
The ratio of 7a/8a; determined by 1H NMR analyses.
2.0 mL of acetone was used.
Ionic liquid 1-butyl-3-methylimidazolium terafluoroborate (300 mg) was added.
Carried out at -30 °C for 96 h.
