Table 3.
Aldol Reaction of Racemic Formylphosphinate Hydrate and Ketonesa
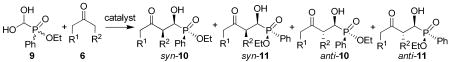 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| entry | 6 | catalyst | 10 and 11 | time (h) | yield (%)b | 10/11c | syn/antid | ee (%)e | ||||
| R1 | R2 | syn-10 | syn-11 | anti-10 | anti-11 | |||||||
| 1f | H | H | 4 | a | 24 | 0 | --- | --- | --- | --- | --- | --- |
| 2f | H | H | 1 | a | 1.5 | 89 | 50:50 | --- | 90g | 79g | --- | --- |
| 3 | H | H | 1 | a | 7 | 91 | 50:50 | --- | 93g | 87g | --- | --- |
| 4f | -(CH2)2- | 1 | b | 24 | 79 | 50:50 | >99:1 | 97 | 62 | --- | --- | |
| 5 | -(CH2)2- | 1 | b | 30 | 83 | 50:50 | 99:1 | 96 | 87 | --- | --- | |
| 6 | -(CH2)3- | 1 | c | 32 | 88 | 50:50 | 35:65 | 98h | 95h | 89h | 93h | |
| 7 | -(CH2OCH2)- | 1 | d | 30 | 91 | 50:50 | 40:60 | 99h | 94h | 26h | 99h | |
Unless otherwise indicated, all reactions were carried out with the racemic formylphosphinate hydrate (0.50 mmol) in dry ketone (0.5 mL) with the catalyst (0.10 mmol, 5 mol %) for the specified reaction time at 0 °C.
Total yield of the inseparable diastereomers (10 and 11) isolated after column chromatography.
Determined by 1H NMR analyses; the value refers to the ratio of the products containing RP and SP stereochemistry.
Determined by 1H NMR analyses, syn and anti refer to the stereochemistry of the two newly generated carbon stereogenic centers.
Enantioselectivity was determined by HPLC analyses.
The reaction was carried out at room temperature.
This compound does not has syn or anti stereochemistry.
The assignments of the ee values to the structures are arbitrary in these cases. Due to partial overlaps of some of the peaks, the error limit of the ee values is considerably larger than normal.
