Table 19.1.
Chemical structures, properties, and theoretical m/z values of nitrohydroxytryptophan and its derivatives by MALDI analysis
| Structures | Comments | Theoretical m/z of the protonated ions |
|---|---|---|
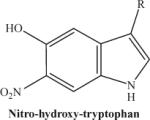 |
The protonated molecular ion is abundantly formed if the synthetic nitrohydroxytryptophan is used. In contrast, the peak intensity will be much lower if using an HPLC eluate from a protein digest. | 266.23 |
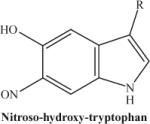 |
Photodecomposition product of nitrohydroxytryptophan following laser exposure: there is a loss of one oxygen atom from the nitro group. The fragmented ion will be predominant if the initial concentration of nitrohydroxytryptophan is low. | 250.23 |
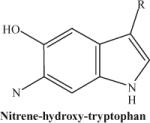 |
Photodecompostion product of nitrohydroxytryptophan following laser exposure: note the loss of two oxygen atoms from the nitro group. This fragmented ion is not observed consistently. | 234.23 |
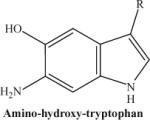 |
The compound obtained after treatment with dithionite. | 236.25 |
