Mutations in the gene coding for the catalytic subunit of the mitochondrial DNA (mtDNA) polymerase γ (POLG1) are associated with a range of clinical syndromes characterized by secondary mtDNA defects, including mtDNA depletion and multiple mtDNA deletions.1 The phenotypic spectrum of POLG1-associated disease ranges from fatal childhood encephalopathy with intractable epilepsy and liver failure (Alpers-Huttenlocher syndrome)2 to late-onset clinical disease affecting a single organ (for a review, see reference 3). We describe a fatal skeletal and visceral myopathy in the neonatal period associated with recessive POLG1 mutations.
Case report.
A newborn boy of healthy nonconsanguineous parents was delivered at 37 weeks’ gestation by cesarean section. His mother (primipara, 32 years old) had been admitted to our hospital 2 weeks previously because of reduced fetal intrauterine movements and polyhydramnios. The child’s birthweight was 2,330 g (<10th percentile), length 47 cm, and head circumference 33.2 cm (25th percentile). He had low-set ears and bilateral clubfoot. Apgar scores were 2, 6, and 7 at 1, 5, and 10 minutes. The child presented with severe hypotonia and generalized muscle weakness, requiring ventilatory assistance and total parenteral nutrition. Weaning failed because of inadequate pulmonary ventilation and respiratory acidosis. Hearing loss was detected by auditory evoked potentials, while cranial MRI showed mildly enlarged ventricles and liquor spaces. Two days after birth, the infant presented with severe abdominal distension with a hypoactive bowel. MRI revealed marked intestinal dilation without mechanical obstruction. Laboratory investigations showed hypoglycemia (27 mg/dL), hypomagnesemia (0.58 mmol/L), and hypokalemia (2.4 mmol/L). Blood lactate was normal (1.3 mmol/L, normal range 0.5–2.2 mmol/L) and liver enzymes were unremarkable. A skeletal muscle biopsy was performed and showed scattered, hypertrophic cytochrome c oxidase (COX)-deficient and succinate dehydrogenase–positive muscle fibers (figure), suggesting a mitochondrial disorder. Molecular genetic studies revealed marked mtDNA depletion in muscle (93% decrease as compared to age-matched controls), while a screen for mtDNA rearrangements within individual COX-positive and COX-deficient fibers4 was negative. We sequenced the entire coding region and intron-exon boundaries of the POLG1 gene, identifying two reported heterozygous missense mutations in compound c.679C>T predicting p.R227W and c.2542G>A predicting p.G848S. Sequencing of parental samples confirmed recessive inheritance.
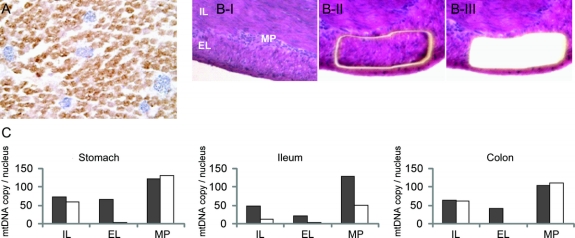
Figure Morpho-molecular features of skeletal muscle and gastrointestinal tract
(A) Combined COX/SDH histochemistry on skeletal muscle biopsy showing numerous hypertrophic COX-deficient muscle fibers (blue). (B) Small intestine wall of POLG1 patient, before (I), during (II), and after (III) laser microdissection of cells from the external layer of muscularis propria. Histologic features are unremarkable. Hematoxylin-eosin, x20. (C) Real-time PCR evaluation of mtDNA amount on microdissected tissue from gastrointestinal wall of patient (white) and one age-matched autopsy control (gray). Data are expressed as the mean value of three repeated measurements. MP = myenteric plexus; IL = internal layer; EL = external layer of muscularis propria.
The infant died at 20 days of respiratory failure. At autopsy, the brain did not show remarkable changes on gross examination. Histology was not informative due to poor preservation of tissue; there was no evidence of neuronal damage in the spinal cord. The liver showed diffuse cholestasis, consistent with total parenteral nutrition; hepatocyte steatosis, necrosis, or liver fibrosis were not observed. The testicles were undescended, while remaining visceral organs were normal except for a marked dilation and thinning of the bowel wall. Despite normal histology, analysis of stomach, ileum, and colon homogenates revealed severe mtDNA depletion (up to 94% decrease; table e-1 on the Neurology® Web site at www.neurology.org). Laser capture microdissection analysis5 revealed that the mtDNA depletion was confined to the muscularis propria, being most prominent in its external layer (figure). Ganglion cells from the myenteric plexus showed milder mtDNA depletion, restricted to the small intestine (figure). There was no mtDNA depletion in liver (not shown).
Discussion.
We describe an infant with a multisystem disorder whose main clinical features were severe skeletal myopathy and visceral dysmotility. Sequencing of the POLG1 gene identified compound heterozygous mutations. Both mutations have been reported previously as recessive, although not together; the p.G848S mutation in patients presenting with PEO, Alpers-Huttenlocher syndrome, and a case with encephalopathy and stroke-like episodes; the p.R227W mutation in Alpers-Huttenlocher syndrome and sporadic PEO (http://tools.niehs.nih.gov/polg).
Children with mutations in POLG1 typically manifest in the first years of life with Alpers-Huttenlocher syndrome2 or a progressive multisystem disorder without liver failure. Combined respiratory chain deficiency due to mtDNA depletion in affected tissues is often observed.3,6 Our patient showed mild cerebral atrophy, yet typical symptoms of Alpers-Huttenlocher syndrome such as intractable seizures and signs of liver dysfunction were not observed. The prominent feature was a severe muscle weakness, with marked mtDNA depletion and COX-deficient muscle fibers, leading to death from respiratory insufficiency. In addition, mtDNA depletion was the likely cause of a visceral myopathy causing hypoperistalsis and intestinal pseudo-obstruction. The molecular features observed in the gastrointestinal tract parallel those recently reported in another autosomal recessive syndrome, mitochondrial neurogastrointestinal encephalomyopathy.5 Based on these findings, the external layer of muscularis propria is confirmed as the most susceptible point of the gastrointestinal tract to develop mtDNA depletion, possibly because of the constitutive low abundance of mtDNA within smooth muscle cells at this site.
ACKNOWLEDGMENT
The authors thank Flaminia Calzolari and Paola Repole for assistance with manuscript preparation.
Supplementary Material
Supplemental data at www.neurology.org
Supported by Telethon Grant GGP06233A, Associazione Serena Talarico per i giovani nel mondo, and the Wellcome Trust (UK).
Disclosure: The authors report no disclosures.
Received August 19, 2008. Accepted in final form December 1, 2008.
Address correspondence and reprint requests to Dr. Giulia d’Amati, Department of Experimental Pathology, Sect. of Pathology, Sapienza University, Policlinico Umberto I, Viale Regina Elena 324, 00161 Rome, Italy; giulia.damati@uniroma1.it
&NA;
- 1.Hudson G, Chinnery PF. Mitochondrial DNA polymerase-gamma and human disease. Hum Mol Genet 2006;15:R244–R252. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari G, Lamantea E, Donati A, et al. Infantile hepatocerebral syndromes associated with mutations in the mitochondrial DNA polymerase-gamma A. Brain 2005;128:723–731. [DOI] [PubMed] [Google Scholar]
- 3.Horvath R, Hudson G, Ferrari G, et al. Phenotypic spectrum associated with mutations of the mitochondrial polymerase gamma gene. Brain 2006;129:1674–1684. [DOI] [PubMed] [Google Scholar]
- 4.He L, Chinnery PF, Durham SE, et al. Detection and quantification of mitochondrial DNA deletions in individual cells by real-time PCR. Nucleic Acids Res 2002;30:e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giordano C, Sebastiani M, De Giorgio R, et al. Gastrointestinal dysmotility in mitochondrial neuro-gastrointestinal encephalomyopathy is caused by mitochondrial DNA depletion. Am J Pathol 2008;173:1120–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Vries MC, Rodenburg RJ, Morava E, et al. Multiple oxidative phosphorylation deficiencies in severe childhood multi-system disorders due to polymerase gamma (POLG1) mutations. Eur J Pediatr 2007;166:229–234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.