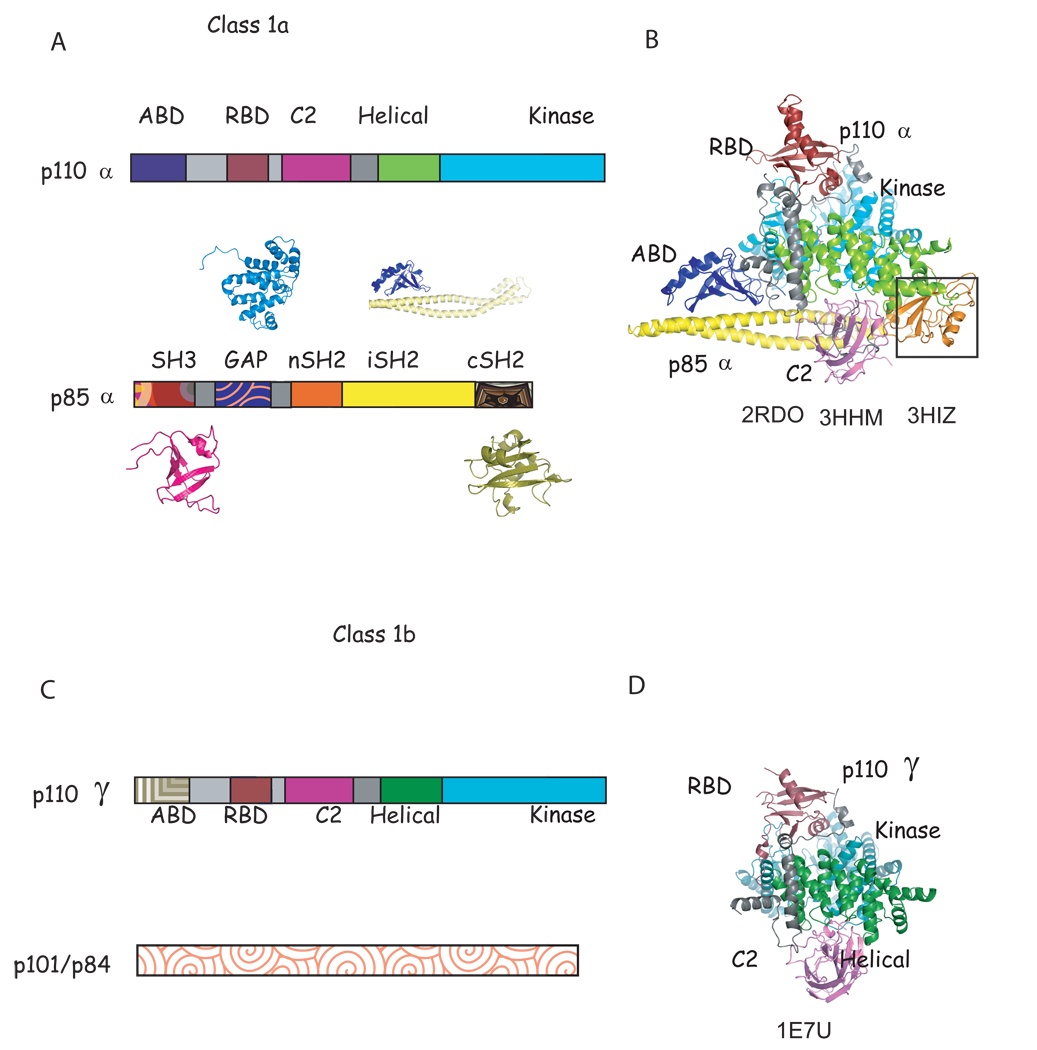
Figure 1. Characteristic of class 1a and known structures of class 1a and class 1b phosphoinositide 3 kinases.
A. Scheme of the domain structure of the heterodimer of class 1a. The structure of all domains of p85α have been determined individually (shown in patterned colors) (SH3: 1PNJ, 2PNI[43]; GAP: 1PBW[18]; nSH2: 2PNA,2PNB[19, 43], 2IUG, 2IUH, 2IUI[20],1OO4[44]; iSH2: 2V1Y[30]; cSH2: 1H90[21],1QAD[45],1BFI[46], 1PIC[47]); although only nSH2 and iSH2 domain structures(solid colors) have been determined as part of a complex with p110α [23, 24]. B. Ribbon diagram of the known structure of the p110α/niSH2 structure. The black box highlights the nSH2 domain observed in the mutant p110α H1047R/ niSH2 (pdb id 3HMM, 3HIZ[24] but not in native structure (pdb id 2RD0, [23]). C. Scheme of the domain structure of class 1b. D. Ribbon modeled of the known domains of the structure of p110γ. Although more than 20 complex structures of p110γ in complex with inhibitors have been published [22, 27, 40, 41, 48–53], the structures of only four (RBD, C2, helical and kinase) out of the five domains have been determined, and there is no published structural information of the regulatory p101/p84. Homologous domains in p110α and γ are colored in B, C, E and F in the same color in different shade.