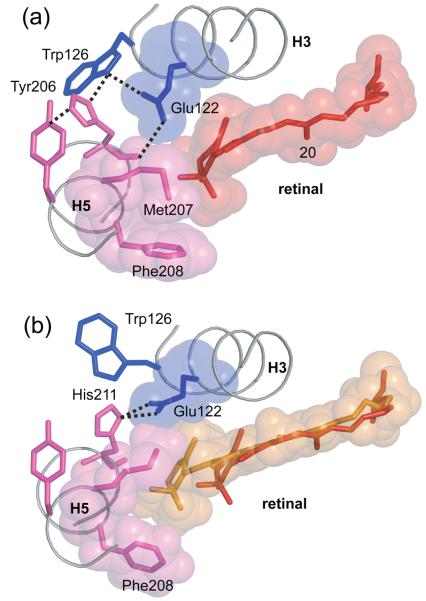
Fig. 5.
Hydrogen bonding of His211 in rhodopsin (A) and Meta II (B). Meta II formation is associated with a change in the hydrogen bonding network centered on His211. In rhodopsin, the backbone carbonyl of His211 forms a hydrogen bond with the side chain of Glu122, while the imidazole side chain of His211 interacts with Trp126. A shift in the position of the retinal β-ionone ring upon activation disrupts the interaction between the backbone carbonyl of His211 and Glu122, and a new hydrogen bond forms with the imidazole side chain δ-nitrogen.