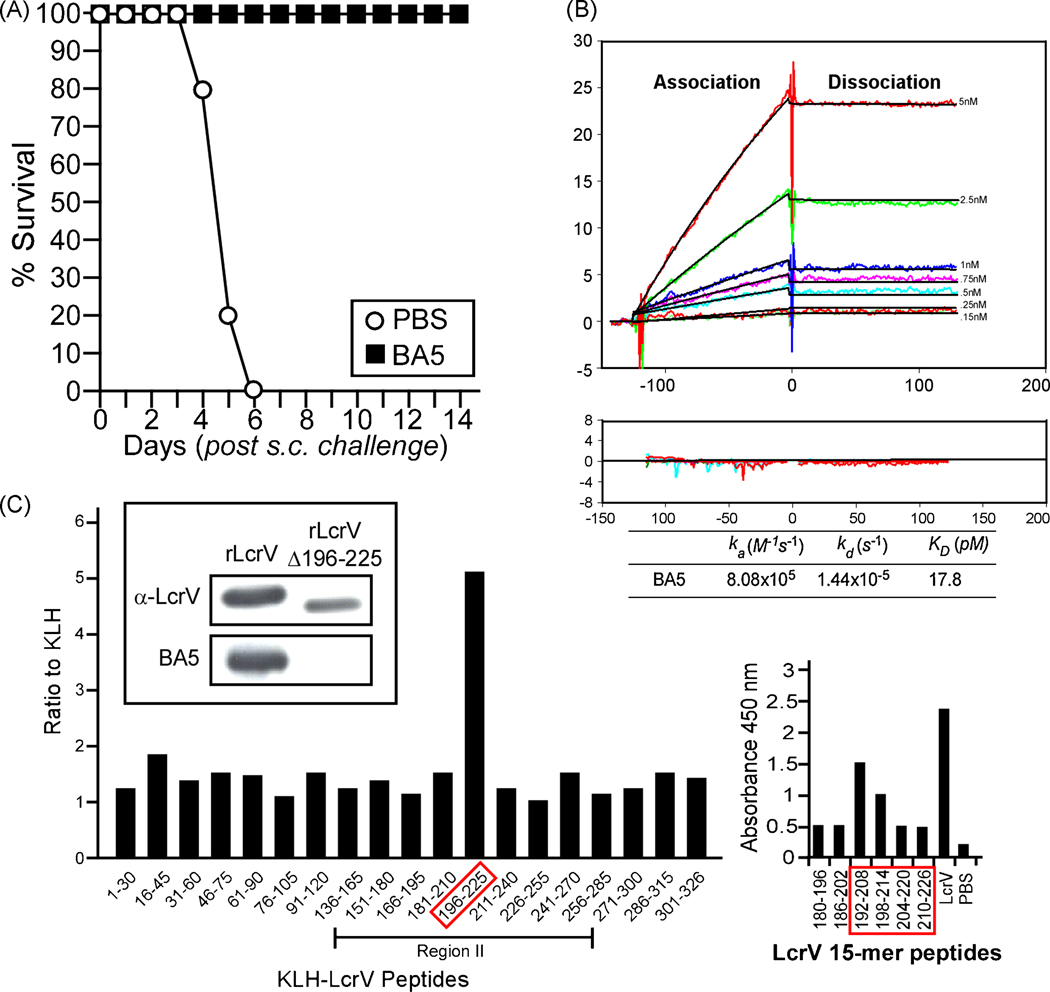
Fig. 1.
Monoclonal antibody (MAb) BA5 binds to rLcrV residues 196 to 225. (A) 8 week old BALB/c mice were injected intraperitoneally with PBS or with 200 µg of LcrV purified monoclonal antibodies one hour prior to subcutaneous challenge with 20 CFU of Yersinia pestis CO92. Mice were monitored for survival for 14 days. (B) Kinetic interaction of full-length LcrV with protective MAb BA5 as visualized by SPR with a BIACORE 3000 machine. BA5 was injected at concentrations ranging from 0.15 to 5 nM (lower to upper curves) at constant flow rate (10µL/min) over 70 RU of immobilized His-tagged LcrV protein or control dextran surface, whose values were subtracted from the signal. The association and dissociation phases were monitored for 120 s by following the change in SPR signal (colored curves) and recording resonance units (RU). Black curves represent the fit of data to a single-site binding model. Kinetic parameters derived from these experiments are listed. Residuals from a single-site binding model indicate excellent fit. (C) Left panel. Peptide fragments (30 residues) spanning the length of LcrV were synthesized and conjugated to KLH. ELISA plates were coated with 100 µL of V peptide fragments or unconjugated KLH (plated at 1µg/mL) and incubated with purified MAb BA5 for 18 hours. Binding was detected with specific secondary antibody conjugated to HRP and read by absorbance at 450 nm. Results are reported as the ratio of binding to the peptide fragment compared to the baseline binding of KLH alone. Region II represents the fragment of LcrV known to contain the epitope for protective MAb 7.3 [26]. Insert: Immunoblots using monoclonal BA5 or rabbit polyclonal antibodies (α-LcrV) to detect recombinant LcrV (rLcrV) or its variant lacking residues 196 to 225 (rLcrVΔ196–225). Right panel. Shorter peptides (15 residues) spanning the length of LcrV ( BEI Resources) were examined for the binding of MAb BA5; immunoreactive peptides, their nearest neighbors in sequence as well as full length and PBS control are shown.