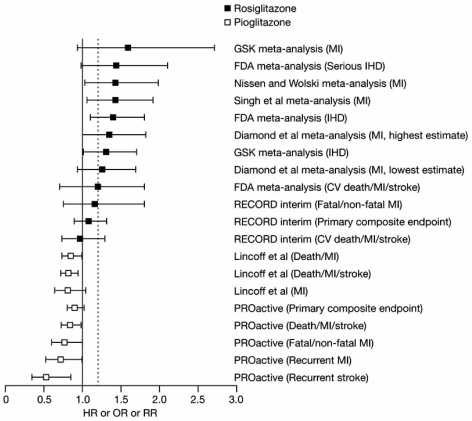
Fig. (3). Available safety data for rosiglitazone and pioglitazone in terms of macrovascular risk relative to comparators.
Data are from meta-analyses, the interim results of the RECORD trial and the PROactive trial. The primary endpoint in RECORD was the composite of hospitalization or death due to CV causes. The primary endpoint in PROactive was the composite of all-cause mortality, MI (incl. silent MI), stroke, ACS, coronary revascularization, major leg amputation and leg revascularization.
The dotted line represents the non-inferiority limit (1.2) for the upper CI in the RECORD study
FDA=(US) Food and Drug Administration; GSK=GlaxoSmithKine; IHD=ischemic heart disease; OR=odds ratio; HR=hazard ratio; RR=relative risk. (No permission required).