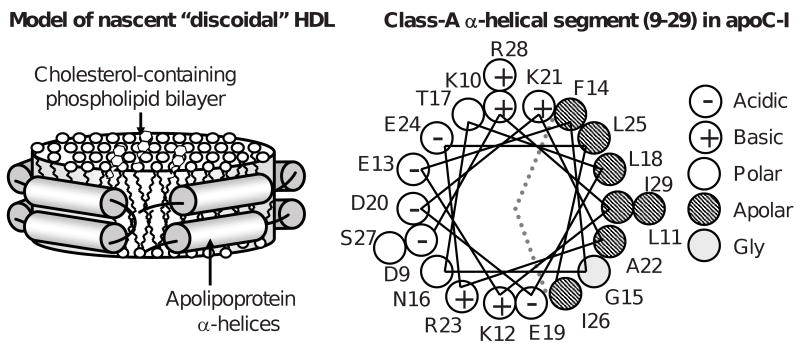
Figure 1.
Cartoon representations of a discoidal HDL and of a class-A apolipoprotein α-helix. (A) Nascent discoidal HDL. Cylinders show protein α-helices. (B) Helix wheel diagram showing the N-terminal segment of human apolipoprotein C-I (residues 9-29), which forms class-A amphipathic α-helix that is the major lipid surface binding motif in apolipoproteins (8). Dotted lines demarcate polar and apolar helical faces. Apolar face is thought to bind lipid surface, while polar face confers particle solubility. Characteristic distribution of charged residues (most of which are located 3-4 positions apart from an oppositely charged group within the helix) allows formation of multiple intra- and interhelical salt bridges that have been proposed to importantly contribute to the stability of the double-belt protein conformation on HDL ((9,10) and references therein).