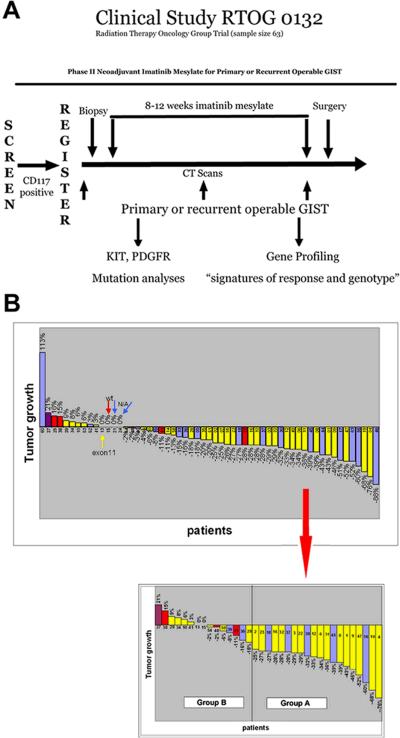
Figure 1. RTOG-S0132 trial design and patient response to IM.
A) Patients with primary or recurrent operable GISTs were screened for KIT (CD117) expression by IHC for eligibility. Prior to IM treatment, a CT was performed and biopsies were collected by core needle aspiration. Patients were then treated with an 8-12 week regimen of IM, followed by cytoreductive surgery. A CT was also performed once during treatment (~4-6 weeks into IM treatment) and immediately prior to surgery. B) (Top) Percentage of tumor growth based on CT measurements taken from the longest cross sectional diameter of the primary GIST or the index metastatic lesion(s) for each RTOG-S0132 patient. (Bottom) Specific samples (pre-, post- or both) used for microarray analysis classified as Group A or B based on the percent of tumor shrinkage/growth visualized by CT. Mutational analysis of most patients was performed and is denoted by color of bar (yellow = KIT exon 11 mutants, red = wild-type GISTs, purple = KIT exon 9 mutants, blue = not enough DNA available for mutational analysis). Group A is defined as ≥25% tumor shrinkage after 8-12 weeks of IM and Group B contains tumors demonstrating <25% tumor reduction, no change, or evidence of tumor enlargement after 8-12 weeks of IM.