Figure 1.
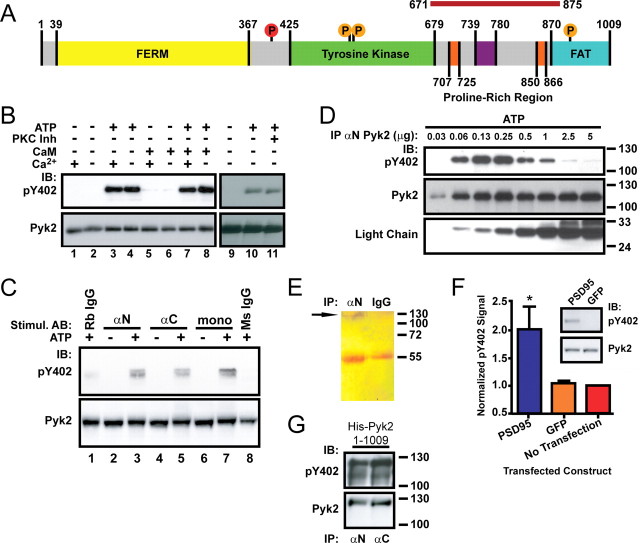
Pyk2 dimerization by antibodies or by PSD-95 induces autophosphorylation. A, Schematic of Pyk2 depicting the FERM, tyrosine kinase, and FAT domains. Proline-rich segments are orange. Src phosphorylation sites are denoted as orange circles, and the autophosphorylation site (Tyr402) is red. The magenta bar on top represents the PSD-95 binding region (residues 671-875). B, Pyk2 was immunoprecipitated (αN Pyk2 antibody) from rat brain cytosol and incubated for 1 h at 4°C in the absence or presence of Mg-ATP, Ca2+ (10 mm), calmodulin (10 μm), or a mixture of PKC inhibitors (200 nm bisindolylmaleimide, 5 μm chelerythrine chloride, and 1 μm calphostin C) before immunoblotting with anti-phospho-Tyr402 (pY402), followed by reprobing with the monoclonal Pyk2 antibody. Autophosphorylation occurred independent of Ca2+/calmodulin (compare lanes 3, 4, 7, 8) or PKC activity (lanes 10, 11). C, Rat brain cytosol was incubated with αN, αC, monoclonal Pyk2 antibodies, or nonspecific rabbit or mouse IgG in the absence or presence of Mg-ATP as indicated and directly analyzed by immunoblotting with anti-phospho-Tyr402 and subsequently the monoclonal Pyk2 antibody. D, Increasing amounts of αN Pyk2 antibody were immobilized on protein A Sepharose, washed, incubated with identical amounts of rat brain cytosol, washed, incubated with Mg2+-ATP for 1 h at 4°C, and processed for immunoblotting with anti-phospho-Tyr402 and subsequently monoclonal Pyk2 antibody and an antibody against the light chain of the immunoprecipitating antibody. E, Immunoprecipitation from brain lysate with antibodies against Pyk2 (αN) or control rabbit IgG was followed by SDS-PAGE and silver staining. The thin band near the top of the blot corresponding to the molecular size of Pyk2 is labeled with an arrow and absent in control precipitates. The bands at 55 kDa are the heavy chains of the antibodies used in the immunoprecipitations. F, PC6-3 cells were transfected with PSD-95 24 h before immunoblotting. Immunosignals were quantified by film densitometry and phospho-Tyr402 values (top of inset) per total Pyk2 signal (bottom of inset) normalized to the no-transfection control. Shown are averages ± SEM of n = 3. *p < 0.05, statistically different from GFP-transfected cells by t test. G, E. coli was transformed with pET28a carrying His-tagged Pyk2 and expression induced with IPTG before extraction and immunoblotting with anti-phospho-Tyr402, followed by probing for total Pyk2. The lower band in the phospho-probing is likely a slightly truncated degradation product and was not visible in the reprobing for total Pyk2 because the reprobing was less sensitive than the original phospho-probing. A–G, All blots are representative of three independent experiments. IB, Immunoblots; IP, immunoprecipitation.