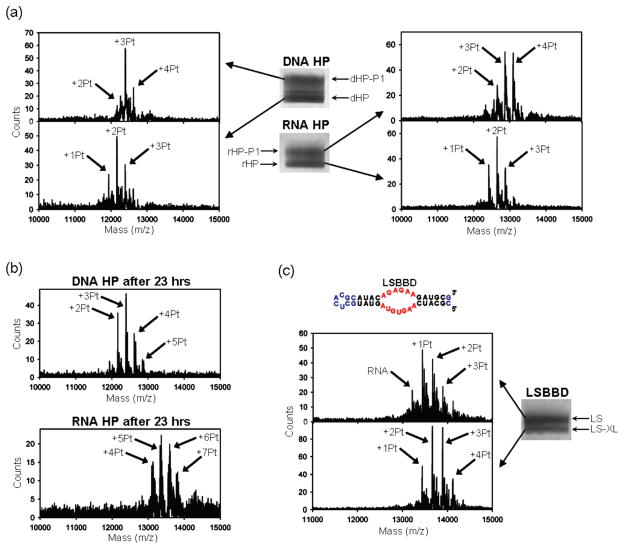
Figure 5.
(a) Positive-ion mode MALDI mass spectra of products following aquated cisplatin treatment of the RNA HP and DNA HP and isolation via dPAGE. Product bands are labeled rHP-P1 and dHP-P1 for RNA HP (rHP) and DNA HP (dHP), respectively. (b) Positive-ion mode MALDI mass spectra of the products of 23 h reactions of RNA HP and DNA HP with cisplatin under the reaction conditions used for Figure 4. (c) Sequence and predicted secondary structure of LSBBD. The BBD internal loop is highlighted in red. Differences in sequence relative to BBD are shown in blue. Image and subsequent MALDI-MS of the two main electrophoretic bands resulting from cisplatin treatment of LSBBD (LS), with LS product band labeled as LS-XL. Conditions: (a) Reactions were performed with 30 μM oligonucleotide, 150 μM aquated cisplatin, 100 mM NaNO3, 1 mM Mg(NO3)2, and 5 mM MOPS (pH 6.8) at 37 °C for 5 h. The bands were separated by 20% dPAGE, stained with methylene blue, and then excised. The MALDI was performed in 3-hydroxypicolinic acid. (c) Same as in (a), except that the reaction contained 90 μM cisplatin.