Summary
Background
We aimed to examine the association between the metabolic syndrome and salt-sensitivity of blood pressure (BP).
Methods
1,906 Chinese aged ≥16 years without diabetes were fed a low-sodium diet (51.3 mmol/day) for 7 days followed by a high-sodium diet (307.8 mmol/day) for an additional 7 days. BP were measured at baseline and at the end of each intervention period using a random-zero sphygmomanometer. Metabolic syndrome was defined as the presence of ≥3 risk factors: abdominal obesity, high triglyceride, low high-density-lipoprotein-cholesterol, elevated BP, and elevated glucose.
Findings
Multivariable-adjusted mean changes (95% confidence intervals) in BP (mmHg) were significantly greater (all p<0.0001) among participants with compared to those without the metabolic syndrome: −7.34 (−8.21, −6.46) versus −5.17 (−5.51, −4.83) for systolic BP and −4.56 (−5.28, −3.85) versus −2.47 (−2.74, −2.19) for diastolic BP during the low-sodium intervention; and 6.51 (5.76, 7.26) versus 4.55 (4.26, 4.84) for systolic BP and 3.25 (2.56, 3.94) versus 1.69 (1.42, 1.96) for diastolic BP during the high-sodium intervention. In addition, compared to those with zero, participants with 4 or 5 risk factors for the metabolic syndrome had a 3.54-fold increased odds (2.05, 6.11) of high salt-sensitivity during the low-sodium intervention and a 3.13-fold increased odds (1.80, 5.43) of high salt-sensitivity during the high-sodium intervention.
Interpretation
These results suggest that the metabolic syndrome significantly enhances BP response to sodium intake. Reduction in sodium intake may be an especially important component in reducing BP among patients with multiple risk factors for the metabolic syndrome.
Keywords: blood pressure, metabolic syndrome, dietary sodium, hypertension, salt sensitivity
Introduction
Hypertension is a major public health problem worldwide because of its high prevalence and consequent increase in risk of vascular disease and premature death (1). Epidemiologic studies and clinical trials have demonstrated that a reduced intake of dietary sodium lowers blood pressure (BP) in both hypertensive and normotensive persons (2,3). BP reduction in response to a decrease in dietary sodium intake, however, may vary considerably among different individuals (4). Identifying persons or subgroups that are more sensitive to dietary sodium intervention has important clinical and public health implications, including the targeting of sodium reduction interventions to those who are most likely to benefit.
Small clinical studies have suggested that insulin resistance may lead to sodium retention and extracellular fluid volume expansion, thereby increasing BP responses to sodium intake (5,6). Since insulin resistance is thought to be the underlying mechanism for the metabolic syndrome, it is likely that individuals with the metabolic syndrome are more sensitive to a dietary sodium intervention. However, the association between the metabolic syndrome and salt-sensitivity of BP has not been well established. We conducted a large dietary intervention feeding study to examine the association between the metabolic syndrome and BP responses to dietary sodium interventions in rural areas of northern China.
Methods
Study population
Details of the study population and methods for the Genetic Epidemiology Network of Salt-Sensitivity (GenSalt) have been published elsewhere (7). In brief, the study was conducted in rural areas of northern China from October 2003 to July 2005. A community-based BP screening was conducted among persons aged 18–60 years in the study villages to identify potential probands and their families. Those with a mean systolic BP between 130–160 mm Hg and/or a diastolic BP between 85–100 mm Hg and no use of antihypertensive medication as well as their siblings, spouses, and offspring were recruited for the study. Individuals who had stage-2 hypertension (BP ≥160/100 mm Hg), secondary hypertension, clinical cardiovascular disease, diabetes (fasting plasma glucose ≥126 mg/dL), chronic kidney disease, current use of antihypertensive or antidiabetic medications or insulin, pregnancy, heavy alcohol consumption, or current use of a low-sodium diet were excluded from the study. A total of 1,906 participants volunteered to take part in the study. Of this group, 25 for whom metabolic risk factor information was missing and 28 who did not complete their dietary interventions were excluded from this analysis.
Data collection
A standard questionnaire was administered by trained staff at the baseline examination to collect information on demographic characteristics, personal and family medical history, and lifestyle risk factors. Three BP measurements were obtained each morning during the 3-day baseline observation period, and on days 2, 5, 6 and 7 of each intervention period by trained and certified observers using a random–zero sphygmomanometer according to a standard protocol. BP was measured with the participant in the sitting position after 5 minutes of rest. In addition, participants were advised to avoid consumption of alcohol, coffee, or tea, cigarette smoking, and exercise for at least 30 minutes prior to their BP measurements. BP observers were blinded to the participant’s dietary intervention. Body weight, height, and waist circumference were measured twice with the participant in light indoor clothing without shoes during their baseline examination. Waist circumference was measured one cm above the participant’s navel during minimal respiration. Overnight (≥8 hours) fasting blood specimens were obtained for measurement of glucose and lipids. Plasma glucose was measured using a modified hexokinase enzymatic method (Hitachi automatic clinical analyser, model 7060, Japan). Concentrations of total cholesterol, HDL-cholesterol, and triglycerides were assessed enzymatically using commercially available reagents. Concentration of LDL-cholesterol was calculated by means of the Friedewald equation for participants who had less than 400 mg/dL triglycerides: LDL cholesterol=total cholesterol–HDL cholesterol–triglycerides/5 (8).
Dietary intervention
Study participants received a low-sodium diet (3 grams of sodium chloride or 51.3 mmol of sodium per day) for 7 days, followed by a high-sodium diet (18 grams of sodium chloride or 307.8 mmol of sodium per day) for an additional 7 days. During both periods of sodium intervention, dietary potassium intake remained unchanged. Dietary total energy intake was varied according to each participant’s baseline energy intake. All foods were cooked without salt, and pre-packaged salt was added to the individual study participant’s meal when it was served by the study staff. To ensure compliance to the intervention program, participants were required to eat their breakfast, lunch and dinner at the study kitchen under the supervision of study staff during the entire study. Study participants were instructed to avoid consumption of any foods that were not provided by study staff members. Three timed urinary specimens (one 24-hour and two overnights) were obtained during the 3 days of baseline examination and the last 3 days of each intervention period (days 5, 6, and 7) to monitor each participant’s compliance with the dietary sodium intervention. The timed overnight urinary excretions of sodium and potassium were converted to 24-hour values based on a formula developed from data obtained in this study. Results from the 24-hour urinary excretion of sodium demonstrated excellent compliance: the mean (standard deviation) 24-hour urinary excretions of sodium and potassium were 242.4 (66.7) mmol and 36.9 (9.6) mmol at baseline, 47.5 (16.0) and 31.4 (7.7) at the end of the low-sodium intervention period, and 244.3 (37.7) and 35.7 (7.5) at the end of the high-sodium intervention period, respectively.
Statistical methods
Mean BP response to the low-sodium intervention was calculated as the mean of the 9 measurements on days 5, 6 and 7 during the low-sodium intervention minus the mean of the 9 measurements at baseline; and response to high-sodium intervention was calculated as the mean of the 9 measurements on days 5, 6 and 7 during the high-sodium intervention minus the mean of the 9 measurements on days 5, 6 and 7 during the low-sodium intervention. In addition, BP response on days 2, 5, 6, and 7 during each intervention period was calculated separately as the difference between the mean of 3 measurements on each day and the mean of the 9 measurements during the previous period. High salt-sensitivity was defined as a mean arterial BP decrease more than 5 mmHg during the low-sodium intervention or an increase of more than 5 mm Hg during the high-sodium intervention (9). Metabolic syndrome was defined as the presence of 3 or more of the following risk factors: waist circumference ≥90 cm in men and ≥80 cm in women; serum triglycerides ≥150 mg/dL; HDL <40 mg/dL in men and <50 mg/dL in women; BP ≥130/85 mm Hg; and plasma glucose ≥100 mg/dL (10).
The adjusted mean BP responses to the low-sodium and high-sodium interventions were compared between participants with and without the metabolic syndrome as well as by the number of metabolic risk factors using multivariate linear regression models. We combined participants with 4 and 5 metabolic syndrome risk factors because there were only a few people with 5 risk factors (n=15). In addition, BP responses on days 2, 5, 6, and 7 during each intervention period were compared between participants with and without metabolic syndrome. Multivariable logistic regression analysis was used to determine the adjusted odds ratio of high salt-sensitivity associated with the metabolic syndrome. In this analysis, the odds ratios of salt-sensitivity was calculated comparing participants with 1, 2, 3, and 4 plus 5 components of the metabolic syndrome to persons with 0 components of the metabolic syndrome. Finally, the adjusted odds ratios of salt-sensitivity were calculated comparing participants with the metabolic syndrome (≥3 components) and without the metabolic syndrome (<3 components). Age, gender, education, physical activity, cigarette smoking, alcohol consumption, body-mass index (BMI), and baseline 24-hour urinary excretion of sodium and potassium were adjusted in multivariable analyses.
Institutional Review Boards or ethics committees at all participating institutes approved the study protocol. Written informed consents for the baseline observation and for the intervention were obtained from each participant prior to data collection or intervention, respectively. This study is registered with ClinicalTrials.gov, number NCT00721721.
Role of the funding source
The NHLBI Project Scientist (Dr. Jaquish) was a member of the study steering committee and was involved in the study design, interpretation of the data, and the writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
The characteristics of study participants by metabolic syndrome status are presented in Table 1. Overall, 15.0% (283/1,881) of study participants had the metabolic syndrome. On average, participants with the metabolic syndrome were older, more likely to be female, and less physically active. As expected, mean systolic and diastolic BP, BMI, waist circumference, fasting triglycerides and glucose levels were significantly higher while HDL-cholesterol was significantly lower in participants with the metabolic syndrome compared with their counterparts without the metabolic syndrome.
Table 1.
Baseline Characteristics of Study Participants by Metabolic Syndrome Status
| Variables | No metabolic syndrome (n = 1,598) | Metabolic syndrome (n = 283) | p-value for difference* |
|---|---|---|---|
| Age, yrs, mean ± SD | 38.3 ± 9.6 | 40.7 ± 8.4 | 0.0001 |
| Men, % (No.) | 54.0 (863) | 46.3 (131) | 0.009 |
| High-school graduate, % (No.) | 14.7 (235) | 13.8 (39) | 0.85 |
| Current drinker, % (No.) | 29.8 (500) | 27.9 (81) | 0.39 |
| Current smoker, % (No.) | 31.3 (476) | 28.6 (79) | 0.36 |
| Physical activity, METS/day†, mean ± SD | 64.5 ± 21.4 | 60.5 ± 19.0 | 0.0008 |
| Blood pressure, mm Hg, mean ± SD | |||
| Systolic | 115.2 ± 13.5 | 126.7 ± 13.6 | <0.0001 |
| Diastolic | 72.4 ± 9.9 | 81.4 ± 9.4 | <0.0001 |
| Body-mass index, kg/m2, mean ± SD | 22.7 ± 2.8 | 26.8 ± 3.0 | <0.0001 |
| Waist circumference, cm, mean ± SD | 78.4 ± 8.8 | 91.1 ± 8.2 | <0.0001 |
| HDL-cholesterol, mg/dL (mmol/L), mean ± SD | 52.8 ± 10.6 (1.37 ± 0.27) | 40.9 ± 10.0 (1.06 ± 0.26) | <0.0001 |
| Triglyceride, mg/dL (mmol/L), mean ± SD | 106.4 ± 55.4 (1.20 ± 0.63) | 220.3 ± 110.4 (2.49 ± 1.25) | <0.0001 |
| Glucose, mg/dL (mmol/L), mean ± SD | 85.7 ± 11.3 (4.76 ± 0.63) | 95.3 ± 17.4 (5.29 ± 0.97) | <0.0001 |
| Urinary excretion, mmol/24-hours, mean ± SD | |||
| Sodium | 239.1 ± 65.3 | 260.9 ± 71.3 | <0.0001 |
| Potassium | 36.8 ± 9.4 | 37.5 ± 10.6 | 0.19 |
Age and gender-adjusted, except for gender which was age-adjusted.
MET, the metabolic equivalent. One MET is equivalent to a metabolic rate consuming 1 kilocalorie per kilogram of body weight per hour.
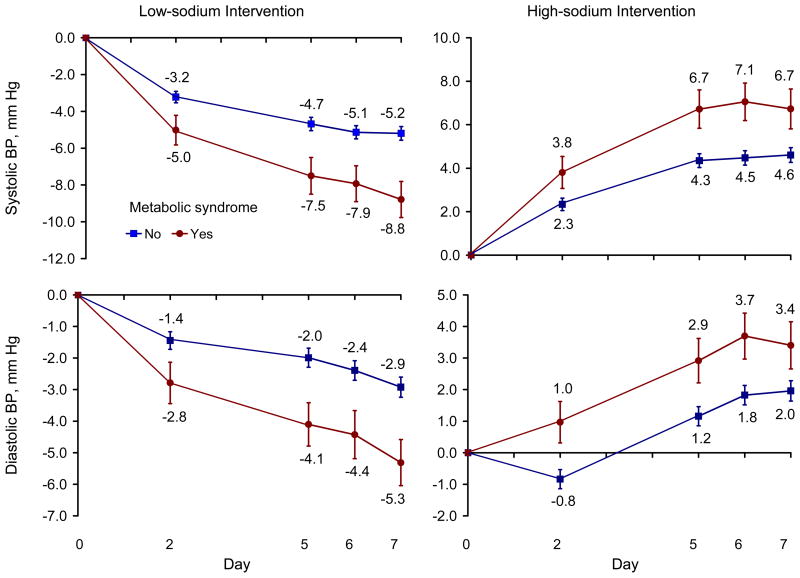
Age and gender-adjusted BP responses were significantly greater among those with the metabolic syndrome compared with those without it on days 2, 5, 6, and 7 of the low-sodium and high-sodium intervention periods (Figure 1). In general, the differences in response increased with longer duration of intervention, especially during the low-sodium intervention. The age and gender-adjusted mean systolic and diastolic BP responses to the low-sodium and high-sodium interventions increased with the number of metabolic risk factors noted (all p<0.0001 for linear trends, Table 2). After adjustment for additional important covariates, the graded association between number of metabolic risk factors and BP response remained highly significant. For example, the multivariable-adjusted mean responses among participants with zero and with 4 or 5 metabolic risk factors during the low-sodium intervention were −4.30 and −9.39 mmHg for systolic and −1.86 and −6.06 mm Hg for diastolic BP, respectively. The corresponding multivariable-adjusted mean responses during the high-sodium intervention were 4.01 and 8.35 mmHg for systolic and 1.43 and 4.41 mm Hg for diastolic BP, respectively. Both the systolic and diastolic BP response to the sodium interventions were significantly greater among those with compared with those without the metabolic syndrome (Table 2).
Figure 1.
Age and gender-adjusted blood pressure responses to low-sodium intervention (left column) and high-sodium intervention (right column) on days 2, 5, 6, and 7 of each intervention period. Blood pressure responses were defined as follows: response to low-sodium = mean of 3 blood pressure measurements on day 2, 5, 6 or 7 during low-sodium intervention – mean of 9 blood pressure measurements at baseline; and response to high-sodium = mean of 3 blood pressure measurements on day 2, 5, 6 or 7 during high-sodium intervention – mean of 9 blood pressure measurements on the last 3 days of low-sodium intervention.
Table 2.
Mean Blood Pressure Responses* (mm Hg) and 95% Confidence Intervals during Low-sodium and High-sodium Intervention According to Number of Metabolic Risk Factors and Metabolic Syndrome Status
| Age-gender-adjusted | Multiple-adjusted† | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Systolic | Diastolic | Systolic | Diastolic | ||||
| Mean responses | 95% Confidence Interval | Mean responses | 95% Confidence Interval | Mean responses | 95% Confidence Interval | Mean responses | 95% Confidence Interval | |
|
Low-sodium Intervention | ||||||||
| Number of metabolic risk factors (No.) | ||||||||
| 0 (n = 607) | −4.26 | (−4.80, −3.72) | −2.04 | (−2.48, −1.60) | −4.30 | (−4.88, −3.72) | −1.86 | (−2.33, −1.39) |
| 1 (n = 586) | −5.61 | (−6.15, −5.07) | −2.45 | (−2.89, −2.01) | −5.62 | (−6.16, −5.08) | −2.40 | (−2.84, −1.96) |
| 2 (n = 382) | −5.59 | (−6.27, −4.92) | −3.19 | (−3.74, −2.65) | −5.64 | (−6.32, −4.96) | −3.32 | (−3.88, −2.76) |
| 3 (n = 200) | −7.00 | (−7.93, −6.07) | −4.10 | (−4.85, −3.34) | −6.94 | (−7.93, −5.95) | −4.37 | (−5.18, −3.56) |
| 4 or 5 (n = 78) | −9.46 | (−10.95, −7.98) | −5.67 | (−6.88, −4.46) | −9.39 | (−10.96, −7.82) | −6.06 | ( −7.34, −4.78) |
| p value for trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| Metabolic syndrome status | ||||||||
| No (n = 1575) | −5.09 | (−5.43, −4.76) | −2.48 | (−2.75, −2.21) | −5.17 | (−5.51, −4.83) | −2.47 | (−2.74, −2.19) |
| Yes (N = 278) | −7.67 | (−8.47, −6.88) | −4.52 | (−5.16, −3.88) | −7.34 | (−8.21, −6.46) | −4.56 | (−5.28, −3.85) |
| p value for difference | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
|
High-sodium Intervention | ||||||||
| Number of metabolic risk factors | ||||||||
| 0 (n = 606) | 4.20 | (3.73, 4.66) | 1.60 | (1.17, 2.03) | 4.01 | (3.51, 4.51) | 1.43 | (0.98, 1.89) |
| 1 (n = 584) | 4.59 | (4.13, 5.06) | 1.39 | (0.96, 1.82) | 4.55 | (4.08, 5.01) | 1.33 | (0.91, 1.76) |
| 2 (n = 378) | 5.11 | (4.52, 5.69) | 2.37 | (1.83, 2.91) | 5.23 | (4.64, 5.82) | 2.51 | (1.97, 3.05) |
| 3 (n = 199) | 5.88 | (5.08, 6.68) | 2.82 | (2.08, 3.56) | 6.13 | (5.28, 6.99) | 3.06 | (2.27, 3.84) |
| 4 or 5 (n = 78) | 8.10 | (6.82, 9.38) | 4.21 | (3.03, 5.39) | 8.35 | (7.01, 9.70) | 4.41 | (3.17, 5.65) |
| p value for trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| Metabolic syndrome status | ||||||||
| No (n = 1568) | 4.57 | (4.28, 4.86) | 1.71 | (1.45, 1.98) | 4.55 | (4.26, 4.84) | 1.69 | (1.42, 1.96) |
| Yes (n = 277) | 6.49 | (5.81, 7.18) | 3.20 | (2.57, 3.83) | 6.51 | (5.76, 7.26) | 3.25 | (2.56, 3.94) |
| p value for difference | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
Mean blood pressure responses were defined as follows: response to low-sodium = mean of 9 measurements on days 5, 6 and 7 of low-sodium diet – mean of 9 measurements from 3 day baseline observation; and response to high-sodium = mean of 9 measurements on days 5, 6 and 7 of high-sodium diet – mean of 9 measurements on days 5, 6 and 7 of low-sodium diet.
Adjusted for age, gender, education, physical activity, cigarette smoking, alcohol consumption, BMI, and 24-hour urinary excretion of sodium and potassium at baseline examination.
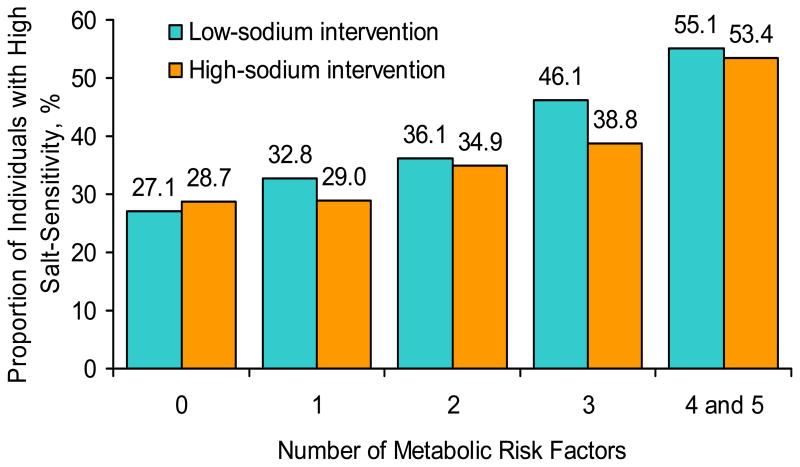
There was a significant and graded association between number of metabolic risk factors and the age and gender-adjusted proportion of study participants with high salt-sensitivity following the low-sodium and high-sodium interventions (both p<0.0001 for linear trend, Figure 2). The dose-response association between number of metabolic risk factors and the odds of high salt-sensitivity remained after adjustment for additional important covariates (Table 3). Compared to those with no metabolic risk factors, participants with 4 or 5 risk factors had a 3.54-fold increased odds of high salt-sensitivity during the low-sodium intervention and a 3.13-fold increased odds of high salt-sensitivity following the high-sodium intervention. In addition, compared to those without the metabolic syndrome (less than 3 risk factors), participants with the metabolic syndrome (3 or more risk factors) had a 92% increased odds of high salt-sensitivity following the low-sodium intervention and a 70% increased odds of high salt-sensitivity following the high-sodium intervention.
Figure 2.
Age and gender-adjusted proportion of high salt-sensitivity during low-sodium and high-sodium intervention. High salt-sensitivity was defined as a mean arterial pressure decrease more than 5 mmHg during the low-sodium intervention or increase more than 5 mm Hg during the high-sodium intervention.
Table 3.
Odds Ratio and 95% Confidence Intervals of High Sodium-sensitivity during Low-sodium and High-sodium Intervention According to Number of Metabolic Risk Factors and Metabolic Syndrome Status
| Variable | Age-gender-adjusted | Multiple-adjusted* | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| Low-sodium Intervention | ||||
| 1 risk factor † | 1.33 (1.03,1.71) | 1.33 (1.03, 1.73) | ||
| 2 risk factors† | 1.54 (1.16,2.04) | <0.0001 | 1.56 (1.16, 2.12) | <0.0001 |
| 3 risk factors† | 2.32 (1.66,3.25) | 2.37 (1.61, 3.49) | ||
| 4 or 5 risk factors† | 3.36 (2.06,5.47) | 3.54 (2.05, 6.11) | ||
| Metabolic syndrome‡ | 2.06 (1.59, 2.68) | <0.0001 | 1.92 (1.43, 2.59) | <0.0001 |
| High-sodium Intervention | ||||
| 1 risk factor† | 1.02 (0.79, 1.32) | 1.04 (0.80, 1.36) | ||
| 2 risk factors† | 1.31 (0.99, 1.74) | <0.0001 | 1.42 (1.05, 1.93) | 0.0002 |
| 3 risk factors† | 1.57 (1.12, 2.22) | 1.74 (1.17, 2.58) | ||
| 4 or 5 risk factors† | 2.90 (1.78, 4.72) | 3.13 (1.80, 5.43) | ||
| Metabolic syndrome‡ | 1.73 (1.33, 2.25) | <0.0001 | 1.70 (1.26, 2.31) | 0.0006 |
Adjusted for age, gender, education, physical activity, cigarette smoking, alcohol consumption, BMI, and 24-hour urinary excretion of sodium and potassium at baseline examination.
Compared with those with 0 metabolic risk factors
Compared with those with 2 or less metabolic risk factors
Sensitivity analyses
In a sensitivity analysis, we excluded the study participants with a baseline BP ≥140/90 mmHg (n=180). The multivariable-adjusted odds ratios (95% confidence intervals) of increased salt-sensitivity associated with the metabolic syndrome were 1.66 (1.19, 2.32) and 1.75 (1.24, 2.46) following the low-sodium and high-sodium interventions, respectively. In addition, we conducted an analysis using a mean arterial pressure change more than 5% during sodium intervention to define high salt-sensitivity. The multivariable-adjusted odds ratios (95% confidence intervals) of high salt-sensitivity associated with the metabolic syndrome were 1.71 (1.27, 2.29) and 1.47 (1.09, 1.99) following the low-sodium and high-sodium interventions, respectively.
Discussion
This large population-based diet-feeding study identified a strong, positive, and significant association between the metabolic syndrome and salt-sensitivity of BP among persons without diabetes. The salt-sensitivity of BP increased progressively with a higher number of metabolic risk factors. This association was independent of age, gender, BMI, physical inactivity, cigarette smoking, alcohol consumption, and baseline dietary intake of sodium and potassium. In addition, the association between the metabolic syndrome and salt-sensitivity remained after the participants with hypertension were excluded.
These findings have important clinical and public health implications. The metabolic syndrome is a common disorder in the general population (11,12). In a national survey, 15.1% of Chinese adults had the metabolic syndrome (12). The metabolic syndrome is associated with an increased risk of cardiovascular disease, diabetes, chronic kidney disease and total mortality (13,14). Salt-sensitivity has also been associated with an increased risk of cardiovascular disease and premature death (15,16). Our study suggests that a reduced intake of dietary sodium may be especially effective in lowering BP among patients with multiple metabolic risk factors.
Our investigation is the first large population-based diet-feeding study to document a strong relationship between the metabolic syndrome and salt-sensitivity of BP. Two previous clinical studies suggested that salt-sensitive hypertension was more common among patients with the metabolic syndrome (17,18). Uzu et al reported that the prevalence of salt-sensitive hypertension was significantly higher in patients with compared to their counterparts without the metabolic syndrome (70.6% versus 36.0%, p=0.02) among 56 Japanese patients (17). In this study, salt-sensitivity was defined as more than a 10% difference in 24-hour ambulatory BP between a one-week period of high-sodium intake (10–12 gram salt/day) and a one-week period of low-sodium intake (1–3 gram salt/day) (17). Hoffmann and Cubeddu reported that systolic and diastolic BP reductions from one-week on a high-salt (18 grams/day) to one-week on a low-salt (2.5 grams/day) diet were significantly greater in participants with compared to those without the metabolic syndrome in 301 participants in Venezuela (18). Compared to these previous studies, our investigation was based on experience in a large population-based sample who participated in a well controlled dietary feeding intervention and careful measurement of BP and other potentially important covariables. The limitations of our study include the relatively short duration of dietary sodium interventions. However, in a recent study among 36 Olivetti Heart Study participants an increased incidence of hypertension during 15-years of follow-up was noted among individuals with high salt-sensitivity of BP as defined by their response to a 3-day period of reduced salt intake (19).
The underlying mechanism of increased salt-sensitivity among individuals with the metabolic syndrome is not fully understood. Insulin resistance and obesity are the most important underlying risk factors for the metabolic syndrome (10,20). Previous studies have suggested that insulin resistance is associated with salt-sensitivity (5,6). Insulin resistance and concomitant compensatory hyperinsulinemia may lead to sodium retention and extracellular fluid volume expansion, thereby increasing BP responses to sodium intake (5). In addition, impaired nitric oxide synthase activity related to insulin-resistance has been implicated in the pathogenesis of salt-sensitive hypertension (21). Furthermore, abnormalities in the sympathetic nervous, renin-angiotensin, kallikrein-kinin and dopamine systems related to insulin resistance seem to contribute to sodium retention and salt-sensitivity (22–24). Previous studies have also noted an association between obesity and salt-sensitivity (25–27). Rocchini and colleagues reported that 60 obese adolescents had a significantly greater change in mean arterial pressure compared to 18 non-obese counterparts after they were changed from a one-week high-salt diet (≥250 mmol of sodium per day) to a one-week low-salt diet (<30 mmol per day) (25). The enhanced salt-sensitivity in obesity may be due to an increased renal tubular reabsorption of sodium in obese individuals (26). In fact, abdominal adiposity and metabolic syndrome in men is associated with increased proximal tubular sodium reabsorption, even after adjustment for BP and insulin resistance (27,28). In our study, the association between the metabolic syndrome and salt-sensitivity remained significant after adjustment for BMI in multivariable analysis. In addition, waist circumference was significantly associated with BP responses to dietary sodium intake. This finding implies that abdominal obesity may be a particularly important factor in determining an individual’s BP response to variation in salt intake.
Previous studies have documented that dietary potassium intake affects salt-sensitivity of BP in human subjects (29). In this feeding study, dietary potassium intake was controlled. In addition, the baseline 24-hour urinary excretion of potassium was adjusted in multivariable models. Previous studies have also indicated that decreased kidney function is related with increased salt-sensitivity (30). In our study, participants with elevated serum creatinine or proteinuria were excluded. Therefore, the association between metabolic syndrome and salt-sensitivity was unlikely due to confounding effects of dietary potassium intake or kidney disease. Furthermore, previous studies have demonstrated that patients with hypertension are more sensitive to a sodium intervention compared to their counterparts who are normotensive (2,3). When we excluded patients with hypertension in a sensitivity analysis, a similarly strong association between the metabolic syndrome and salt-sensitivity of BP persists.
In conclusion, our study suggests that the metabolic syndrome is significantly and independently related to salt-sensitivity of BP, with a graded relationship between the number of metabolic risk factors and salt-sensitivity of BP. Our findings indicate that a reduced intake of sodium may be particularly beneficial in individuals with the metabolic syndrome. Additional intervention studies are warranted to examine the effect of prevention and treatment of metabolic risk factors on salt-sensitivity of BP.
Acknowledgments
The Genetic Epidemiology Network of Salt Sensitivity (GenSalt) is supported by a cooperative agreement project grant (U01HL072507) from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland.
Appendix
The GenSalt Study Steering Committee: Dongfeng Gu, Jiang He (Chair), James E Hixson, Cashell E Jaquish, Depei Liu, DC Rao, Paul K Whelton, and Zhijian Yao.
GenSalt Collaborative Research Group:
Tulane University Health Sciences Center, New Orleans, USA: Jiang He (PI), Lydia A Bazzano, Chung-Shiuan Chen, Jing Chen, Mei Hao, Lee Hamm, Tanika Kelly, Paul Muntner, Kristi Reynolds, Wenjie Yang, and Qi Zhao.
Washington University School of Medicine, St Louis, USA: DC Rao (PI), Matthew Brown, Charles Gu, Hongyan Huang, Treva Rice, Karen Schwander, and Shiping Wang.
University of Texas Health Sciences Center at Houston: James E Hixson (PI) and Lawrence C Shimmin.
Loyola University Health System and Medical Center; Paul K. Whelton
National Heart, Lung, and Blood Institute: Cashell E. Jaquish
Chinese Academy of Medical Sciences, Beijing, China: Dongfeng Gu (PI), Jie Cao, Jichun Chen, Jingping Chen, Zhenhan Du, Jianfeng Huang, Hongwen Jiang, Jianxin Li, Xiaohua Liang, Depei Liu, Xiangfeng Lu, Donghua Liu, Qunxia Mao, Dongling Sun, Hongwei Wang, Qianqian Wang, Xigui Wu, Ying Yang, and Dahai Yu.
Shandong Academy of Medical Sciences, Shandong, China: Fanghong Lu (PI), Zhendong Liu, Shikuan Jin, Yingxin Zhao, Shangwen Sun, Shujian Wang, Qengjie Meng, Baojin Liu, Zhaodong Yang, and Chuanrui Wei.
Shandong Center for Diseases Control and Prevention, Shandong, China: Jixiang Ma (PI), Jiyu Zhang, and Junli Tang.
Zhengzhou University: Dongsheng Hu, Hongwei Wen, Chongjian Wang, Minghui Shen, Jingjing Pan, and Liming Yang.
Xinle Traditional Chinese Medicine Hospital, Hebei, China: Xu Ji (PI), Rongyan Li, Haijun Zu, and Junwei Song.
Ganyu Center for Disease Control and Prevention: Delin Wu (PI), Xushan Wang, and Xiaofeng Zhang.
Xi’an Jiaotong University, Shanxi, China: Jianjun Mu (PI), Enrang Chen, Fuqiang Liu, and Guanji Wu.
Chinese National Human Genome Center at Beijing: Zhi-Jian Yao (PI), Shufeng Chen, Dongfeng Gu, Hongfan Li, Laiyuan Wang, and Penghua Zhang.
Footnotes
Contributors
J Chen, Gu, Rao, Jaquish, Hixson, Rice, Hamm, Whelton, and He initiated and designed the study. Gu, Huang, J-C Chen, Lu, Hu, and He supervised the recruitment of participants and acquisition of data. C Chen analyzed the data under supervision of J Chen and He. J Chen, Gu, Rao, Jaquish, Hixson, Rice, Kelly, Hamm, Whelton and He interpreted the results. J Chen and He wrote the first draft of the manuscript. J Chen, Gu, Rao, Kelly, Whelton, and He revised the report. All authors saw and approved the final version of the manuscript.
Conflict of interest statement
We declare that we have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, et al. Primary Prevention of Hypertension. Clinical and Public Health Advisory from the National High Blood Pressure Education Program. JAMA. 2002;288:1882–1888. doi: 10.1001/jama.288.15.1882. [DOI] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 4.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 5.Shimamoto K, Hirata A, Fukuoka M, Higashiura K, Miyazaki Y, Shiiki M, Masuda A, Nakagawa M, Iimura O. Insulin sensitivity and the effects of insulin on renal sodium handling and pressor systems in essential hypertensive patients. Hypertension. 1994;23:129–33. doi: 10.1161/01.hyp.23.1_suppl.i29. [DOI] [PubMed] [Google Scholar]
- 6.Galletti F, Strazzullo P, Ferrara I, Rivellese AA, Gatto S, Mancini M. Salt sensitivity of essential hypertensive patients is related to insulin resistance. J Hypertens. 1997;15:1485–91. doi: 10.1097/00004872-199715120-00017. [DOI] [PubMed] [Google Scholar]
- 7.GenSalt Collaborative Research Group. GenSalt: rationale, design, methods and baseline characteristics of study participants. J Hum Hypertens. 2007;21:639–46. doi: 10.1038/sj.jhh.1002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of lowdensity lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 9.Sullivan JM. Salt sensitivity. Definition, conception, methodology, and long-term issues. Hypertension. 1991;17(suppl I):I-61–I-68. doi: 10.1161/01.hyp.17.1_suppl.i61. [DOI] [PubMed] [Google Scholar]
- 10.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 11.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–59. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 12.Gu DF, Reynolds K, Wu X, Chen J, Duan X, Reynolds RF, Whelton PK, He J. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet. 2005;365:1398–405. doi: 10.1016/S0140-6736(05)66375-1. [DOI] [PubMed] [Google Scholar]
- 13.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–16. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, Whelton PK, He J. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140:167–74. doi: 10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- 15.Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997;350:1734–7. doi: 10.1016/S0140-6736(97)05189-1. [DOI] [PubMed] [Google Scholar]
- 16.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–32. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 17.Uzu T, Kimura G, Yamauchi A, Kanasaki M, Isshiki K, Araki S, et al. Enhanced sodium sensitivity and disturbed circadian rhythm of blood pressure in essential hypertensive patients with metabolic syndrome. J Hypertens. 2006;24:1626–1632. doi: 10.1097/01.hjh.0000239299.71001.77. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann IS, Cubeddu LX. Increased blood pressure reactivity to dietary salt in patients with the metabolic syndrome. J Human Hypertens. 2007;21:438–444. doi: 10.1038/sj.jhh.1002153. [DOI] [PubMed] [Google Scholar]
- 19.Barba G, Galletti F, Cappuccio FP, Siani A, Venezia A, Versiero M, Della Valle E, Sorrentino P, Tarantino G, Farinaro E, Strazzullo P. Incidence of hypertension in individuals with different blood pressure salt-sensitivity: results of a 15-year follow-up study. J Hypertens. 2007;25:1465–71. doi: 10.1097/HJH.0b013e3281139ebd. [DOI] [PubMed] [Google Scholar]
- 20.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 21.Tolins JP, Shultz PJ. Endogenous nitric oxide synthesis determines sensitivity to the pressor effect of salt. Kidney Int. 1994;46:230–6. doi: 10.1038/ki.1994.264. [DOI] [PubMed] [Google Scholar]
- 22.Strazzullo P, Barbato A, Vuotto P, Galletti F. Relationships between salt sensitivity of blood pressure and sympathetic nervous system activity: a short review of evidence. Clin Exp Hypertens. 2001;23:25–33. doi: 10.1081/ceh-100001194. [DOI] [PubMed] [Google Scholar]
- 23.Fujita T. Spotlight on renin. The renin system, salt-sensitivity and metabolic syndrome. J Renin Angiotensin Aldosterone Syst. 2006;7:181–3. doi: 10.3317/jraas.2006.029. [DOI] [PubMed] [Google Scholar]
- 24.Chan TY, Critchley JA, Ho CS, Tomlinson B, Chan JC, Poon EW, Lee ZS, Critchley LA, Swaminathan R. Renal kallikrein-kinin system, but not renal dopamine system, mediates the natriuretic response to intravenous saline infusion in healthy Chinese subjects. J Auton Pharmacol. 2000;20:37–45. doi: 10.1046/j.1365-2680.2000.00160.x. [DOI] [PubMed] [Google Scholar]
- 25.Rocchini AP, Key J, Bondie D, Chico R, Moorehead C, Katch V, Martin M. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N Engl J Med. 1989;321:580–5. doi: 10.1056/NEJM198908313210905. [DOI] [PubMed] [Google Scholar]
- 26.Hall JE. Mechanisms of abnormal renal sodium handling in obesity hypertension. Am J Hypertens. 1997;10:49S–55S. [PubMed] [Google Scholar]
- 27.Strazzullo P, Barba G, Cappuccio FP, Siani A, Trevisan M, Farinaro E, Pagano E, Barbato A, Iacone R, Galletti F. Altered renal sodium handling in men with abdominal adiposity: a link to hypertension. J Hypertens. 2001;19:2157–64. doi: 10.1097/00004872-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Strazzullo P, Barbato A, Galletti F, Barba G, Siani A, Iacone R, D’Elia L, Russo O, Versiero M, Farinaro E, Cappuccio FP. Abnormalities of renal sodium handling in the metabolic syndrome. Results of the Olivetti Heart Study. J Hypertens. 2006;24:1633–9. doi: 10.1097/01.hjh.0000239300.48130.07. [DOI] [PubMed] [Google Scholar]
- 29.Morris RC, Jr, Sebastian A, Forman A, Tanaka M, Schmidlin O. Normotensive salt sensitivity: effects of race and dietary potassium. Hypertension. 1999;33:18–23. doi: 10.1161/01.hyp.33.1.18. [DOI] [PubMed] [Google Scholar]
- 30.Chiolero A, Wurzner G, Burnier M. Renal determinants of the salt sensitivity of blood pressure. Nephrol Dial Transplant. 2001;16:452–8. doi: 10.1093/ndt/16.3.452. [DOI] [PubMed] [Google Scholar]