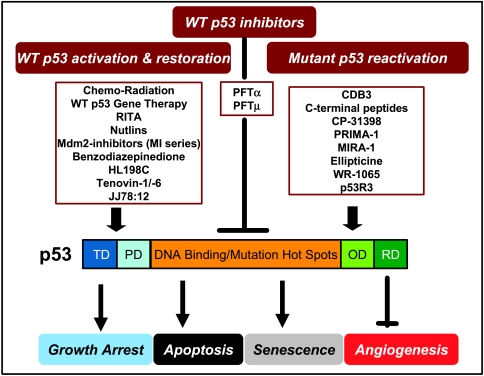
Figure 1.
Current approaches for p53 targeting: p53, the “guardian of the genome,” consists of 393 amino acids with four distinct functional domains. The transactivation domain (TD) and proline-rich domain (PD) is located at the N-terminus, the DNA binding andmutation hot spots domain at the central of themolecule, whereas the oligomerization domain (OD) and regulatory domain (RD) at the C-terminus. On activation, p53 plays a pivotal role in tumor suppression by inducing growth arrest, apoptosis, and senescence, as well as by blocking angiogenesis. Wild-type p53 also confers the sensitivity of cancer cells to chemoradiation. Thus, p53 becomes an appealing therapeutic target for anticancer drug discovery. As illustrated in the figure, three classes of p53 targeting compounds have been identified and characterized. The first class are the compounds that activate or restore wild-type p53 function and can be used in human cancers harboring a wt p53. The second class of compounds reactivates and rescues the mutant p53 with an application in human cancers carrying a p53 mutation. The third class is capable of inhibiting wt p53 and can be used during chemoradiation to block p53 activation in normal cells, thus reducing cytotoxicity.