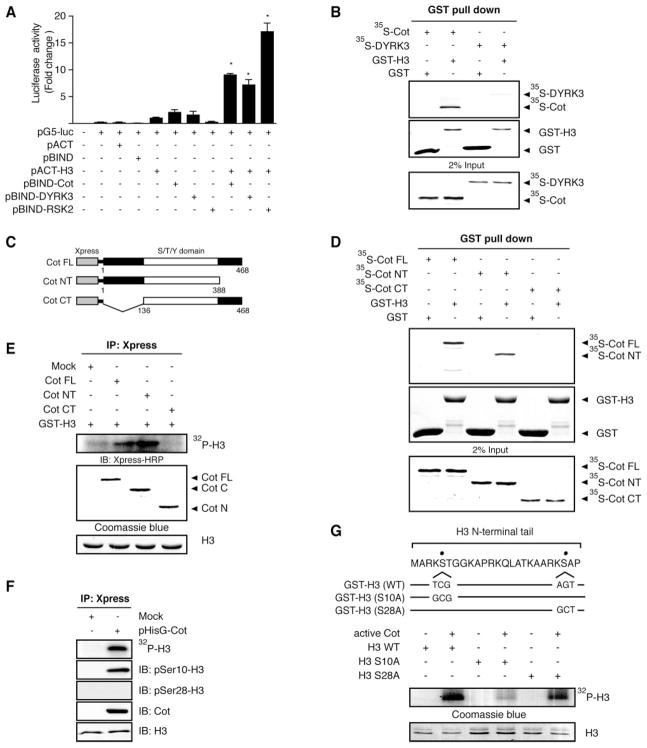
Figure 1.
Cot binds with histone H3. A) Direct interaction of Cot with histone H3 as determined by the M2H assay. For a negative control, pACT or pBIND plasmids were transfected along with the pG5-luc reporter plasmid into NIH3T3 cells (18,000 cells/ml). For a positive control, histone pACT-H3 and pBIND-RSK2 or pBIND-DYRK3 were transfected with the pG5-luc reporter plasmid into NIH3T3 cells. Histone pACT-H3 and/or pBIND-Cot were cotransfected with the pG5-luc plasmid to confirm the interaction of Cot with histone H3. After a 36 h incubation, firefly luciferase activity was determined in the cell lysates and normalized against Renilla luciferase activity. All experiments were performed at least twice with triplicate samples and are depicted as the means ± se. An asterisk (*) indicates a significant increase in activity compared with negative control, pACT-H3 only (P<0.05). The data were recorded as a relative fold change in luciferase activity as measured by a Luminoskan Ascent plate reader (Thermo Electron Corp., Helsinki, Finland). B) In vitro binding with an 35S-labeled Cot protein and a GST-histone H3 fusion protein. The cDNA of Cot or DYRK3 was translated in vitro, then the 35S-Cot or 35S-DYRK3 (positive control) proteins were mixed with GST-histone H3 and a pulldown assay was performed. Proteins were visualized by autoradiography. Input represents 2% of the material used for the in vitro binding assays. C) Schematic diagram of full-length Xpress-Cot (1–468, Cot FL), the N-terminal (1–368) fragment of Xpress-Cot (Cot NT), or the C-terminal (136–468) fragment of Xpress-Cot (Cot CT). D) In vitro interactions of the 35S-labeled full-length Cot (Cot FL), the N-terminal fragment of Xpress-Cot (Cot NT) or the C-terminal fragment of Xpress-Cot (Cot CT) with GST only or GST-H3. E) In vitro phosphorylation of GST-H3 by Cot FL, Cot NT, or Cot CT, which were immunoprecipitated with anti-Xpress, from Cot-overexpressing HEK293 cells. F) In vitro phosphorylation of histone H3 by Cot (top panel) purified from Cot-overexpressing HEK293 cells was detected by immunoblotting with anti-phospho-histone H3 (Ser-10) or anti-phospho-histone H3 (Ser-28). Overexpression of Cot was detected with anti-Xpress in cells transiently transfected with Xpress-Cot (fourth panel). Total histone H3 levels are shown as loading controls (bottom panel). “Mock” denotes a pcDNA4/hisMaxA-transfected control cell line. IB, immunoblotting; IP, immunoprecipitation. G) Schematic diagrams of histone H3 mutation of Ser-10 to alanine (S10A) or mutation of Ser-28 to alanine (S28A) (upper panel). GST pulldown proteins histone H3 wild-type (WT) or histone H3 mutants, S10A or S28A, were incubated with active Cot (1 μg) for 60 min at 30°C for an in vitro kinase assay and the 32P-labeled histone H3 was visualized by autoradiography. For visualizing equal loading of protein, total histone H3 was detected by Coomassie blue (bottom panel). The figures are representative of at least three separate experiments that yielded similar results.