Abstract
Human bowel movements usually occur during the day and seldom during the night, suggesting a role for a biological clock in the regulation of colonic motility. Research has unveiled molecular and physiological mechanisms for biological clock function in the brain; less is known about peripheral rhythmicity. This study aimed to determine whether clock genes such as period 1 (per1) and period2 (per2) modulate rhythmic changes in colonic motility. Organ bath studies, intracolonic pressure measurements, and stool studies were used to examine measures of colonic motility in wild-type and per1per2 double-knockout mice. To further examine the mechanism underlying rhythmic changes in circular muscle contractility, additional studies were completed in neuronal nitric oxide synthase (nNOS) knockout mice. Intracolonic pressure changes and stool output in vivo, and colonic circular muscle contractility ex vivo, are rhythmic with greatest activity at the start of night in nocturnal wild-type mice. In contrast, rhythmicity in these measures was absent in per1per2 double-knockout mice. Rhythmicity was also abolished in colonic circular muscle contractility of wild-type mice in the presence of Nω-nitro-l-arginine methyl ester and in nNOS knockout mice. These findings suggest that rhythms in colonic motility are regulated by both clock genes and a nNOS-mediated inhibitory process and suggest a connection between these two mechanisms.
Keywords: biological rhythms, clock genes, circadian, nNOS
translational highlights Bowel movements and function demonstrate daily rhythms. This study uses a mouse model to demonstrate that daily rhythms in colonic pressure, smooth muscle contractility, and stool output require “clock” genes and also depend on nNOS. These findings have translational implications for better understanding of the disruption in bowel function associated with shift work or changes in time zone.
Circadian timekeeping is a fundamental feature of life; it is a process in organisms ranging from eubacteria to humans in processes ranging from sleep and wakefulness to feeding and gastrointestinal processes (3, 9, 21). By and large, healthy people have bowel movements during the day, frequently following awakening or following a meal. They rarely occur during the night. Ambulatory pressure recordings from healthy individuals demonstrate maximal colonic activity during the day, especially following awakening, with minimal activity during the night (19). Disruption of daily rhythms, such as those that occur with shift work or time zone traveling, has been associated with gastrointestinal symptoms such as abdominal bloating, abdominal pain, diarrhea, or constipation (4–6, 16). These observations strongly suggest that there is a functional link between our circadian clock and colonic motility.
The molecular bases for biological rhythms are believed to be formed by so-called “clock genes.” These transcription factors comprise “positive elements” clock and Bmal1, whose protein products CLOCK and Bmal1 dimerize and stimulate the transcription of the “negative elements” period 1–3 (per1–3) and cryptochrome 1–2 (cry1–2). The per and cry gene products in turn are translated, oligomerize, and feed back to inhibit the actions of the CLOCK:Bmal1 dimers. These molecular mechanisms have been reviewed extensively elsewhere (3).
Physiologically, a central pacemaker is located within the suprachiasmatic nucleus (SCN) of the hypothalamus. Destruction of the SCN abolishes overt circadian rhythms; transplantation of SCN tissue or cells into arrhythmic SCN-lesioned rodents restores rhythmicity; and the SCN expresses rhythms of electrical activity, metabolism, and clock gene expression in vivo and in vitro, implying that clock gene expression in the SCN controls downstream rhythms (18). However, clock genes are rhythmically expressed in peripheral tissues as well (11, 22, 28).
Previous studies from our laboratory demonstrated that clock genes are expressed in epithelial cells and within neurons of the myenteric plexus of the mouse colon (11). In addition, we have shown that subsets of the mouse colon transcriptome are rhythmically expressed, including genes that play an important role in the regulation of colonic motility such as neuronal nitric oxide synthase (nNOS) (12). On the basis of these findings, we have hypothesized that the colon may possess time-keeping capability that allows for the physiological adaptation of colonic motility to environmental stimuli and to physiological events within the body.
To investigate the role of clock genes in the regulation of colonic motility, we studied colonic motility in wild-type (WT) mice and in mice in which a subset of the clock genes [period 1 (per1) and period (per2)] was knocked out. These measures consisted of a combination of ex vivo (colonic circular muscle contractility) and in vivo (intracolonic pressure changes, stool output) methods. Our findings suggest that measures of colonic motility follow a rhythmic pattern that is driven by the molecular circadian clock.
METHODS
Animals
Per1, per2, and per1per2 knockout 129/Sv mice were obtained from the laboratory of Dr. Vincent M. Cassone. These mice were derived from founders obtained from Dr. David Weaver (University of Massachusetts). nNOS knockout mice, B6.129S4 Nos1tmP1h/J, were purchased from Jackson Laboratories (Bar Harbor, ME). Male mice with body weights of 30–35 g were used for all experiments. Experimental protocols were approved by the Institutional Animal Care and Use Committee in accordance with the guidelines provided by the National Institutes of Health.
Experiments
Mice were maintained on a 12-h light:12-h dark (LD) cycle prior to all experiments. Time is indicated in LD cycles using Zeitgeber time (ZT) whereby ZT0 refers to the time at which lights came on and ZT12 refers to the time that lights went off. Studies were completed every 6 h or every 12 h starting at ZT1 for all experiments. For a rhythm to be considered circadian, it has to persist in constant environmental conditions. Therefore, a subset of experiments was completed in constant darkness (DD). To remove the effect of light, mice were released into DD for a total of 120 h prior to the start of the experiment. In DD, circadian time was referenced to the previous ZT such that the beginning of the original light cycle was labeled ZT0 and the beginning of original dark cycle was labeled ZT12. Although it would have been preferable to refer to circadian time relative to the animals' internal time, the absence of a phase reference from wheel running, for example, was impractical. Therefore, ZT was employed as a compromise.
Organ Bath Studies
Organ bath studies were completed by using an EZ-bath-8 channel organ bath system (GlobalTown MicroTech, Sarasota, FL). Mice were anesthetized with xylazine 10 mg/kg ip and ketamine 80 mg/kg ip. The proximal and distal colon were removed and placed in Krebs at ZT1 (subjective day) and at ZT13 (subjective night). Organ bath studies were completed immediately following resection. Two colonic rings, 3 mm wide, were cut from each site of the colon and mounted in a 5-ml bath filled with oxygenated Krebs solution (130 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 10 mM glucose, 13 mM sucrose, 20 mM HEPES, pH 7.3) at 37°C and equilibrated for 60 min. The bath solution was changed every 15 min until the frequency of spontaneous contractions stabilized. Rings were stretched to 0.5-g tension. Increasing doses of acetylcholine were administered to obtain complete dose-response curves of circular muscle contraction. In experiments with combined addition of antagonists, nitric oxide synthase inhibitor Nω-nitro-l-arginine methyl ester (l-NAME) 100 μM or tetrodotoxin (TTX) 1 μM were added to the bath 30 min prior to the addition of the first dose of acetylcholine, during the administration of increasing doses of acetylcholine, and with the washes in between the various doses of acetylcholine. The dry weight of each muscle ring was measured after completion of the experiment.
Stool Studies
Stool pellets were collected every 4 h and dry weight was measured. Data were expressed as average stool output in milligrams ± standard error.
Telemetry
Colonic contractility in WT and per1per2 double-knockout mice was measured by using an ambulatory telemetry system previously reported for quantifying gastric contractility (7). Mice were anesthetized with isoflurane gas anesthesia. Following a midabdominal laparotomy, the distal portion of the colon was exposed and a pressure catheter connected to a transducer (Data Sciences, St. Paul, MN) was introduced into the distal colon and sutured in place with a 10 mm 3/8″-circle round-bodied needle and 6-0 size braided multifilament silk thread. The transducer housing was fixed within the peritoneal cavity. Five days postsurgery, mice were placed in individual cages on receivers that acquire radiofrequency signals transmitted by the transducer. Signals were converted to intracolonic pressure recordings from individual mice by a PowerLab data-acquisition system and analyzed with LabChart6 software (ADInstruments, Colorado Springs, CO).
Data Analysis
Organ bath studies.
The contractile response was measured as the area under the curve (AUC) in grams ∗ seconds for 2 min after the administration of the agonist minus a 2-min baseline AUC. Results were expressed as the average AUC in grams times seconds ± standard error of responses of individual muscle rings. The n value represents the number of animals. Comparisons between contractile responses were completed by ANOVA followed by Tukey's multiple comparison testing (Sigmastat); significance levels were set at P < 0.05. Cosinor analysis was used to determine whether the circular muscle response to acetylcholine response followed a statistically significant circadian rhythm as previously described (11). The original data expressed at AUC (g·s) at each dose was expressed as a percentage of the overall mean (at that dose, across the 4 time points). Percentage rhythm refers to the equivalent of a correlation coefficient that determines how close an observed rhythm is to a cosine curve.
Telemetry.
Intracolonic pressure tracings were analyzed by measurement of the AUC for 12-h time periods. Comparisons between AUC night vs. day were completed by a Student's t-test; significance levels were set at P < 0.05.
Stool studies.
Comparisons of stool output among experimental groups were by using a Student's t-test; significance levels were set at P < 0.05.
RESULTS
Measures of Colonic Motility in WT Mice
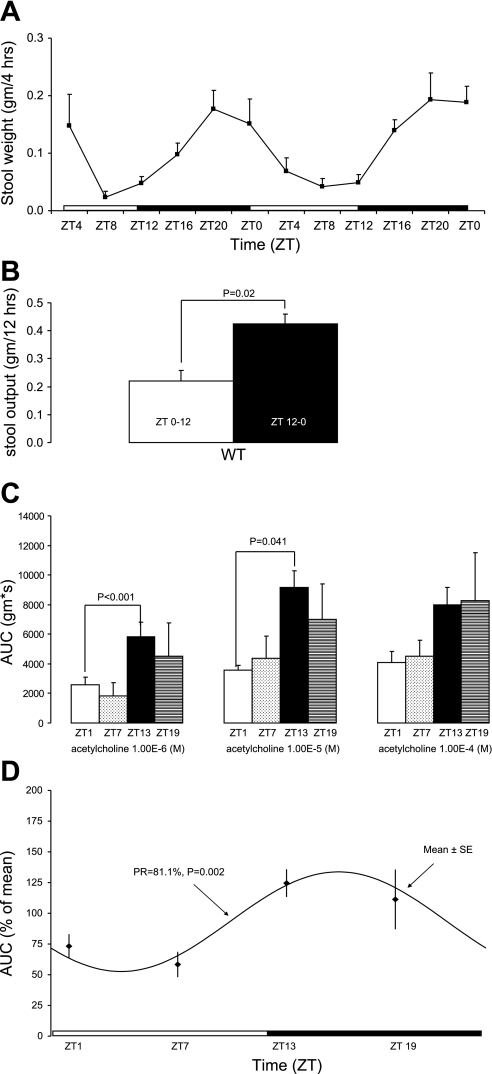
Stool output in WT mice maintained on a regular LD with ad libitum access to feeding is rhythmic (Fig. 1A) with most of the stool output occurring at night (Fig. 1B). Colonic circular muscle contractile response to acetylcholine varied over the time of day. Colonic circular muscle contractility in WT mice is greater at the beginning of the dark cycle (ZT13) compared with the beginning of the light cycle (ZT1) at various doses of acetylcholine (Fig. 1C). Cosinor analysis indicated colonic circular muscle contractile response to acetylcholine was statistically significant in its rhythmicity (Fig. 1D).
Fig. 1.
Measures of colonic motility are rhythmic in wild-type (WT) mice on a light-dark cycle. A: stool output in WT mice (n = 3). ZT, Zeitgeber time, whereby ZT0 refers to the time at which lights came on and ZT12 refers to the time that lights went off. B: total stool output during light (ZT0–12) and dark cycle (ZT12–0). C: colonic circular muscle contractility in WT mice at ZT1 (n = 4), ZT7 (n = 6), ZT13 (n = 8), and ZT19 (n = 5) expressed as area under the curve (AUC; g·s) ± SE. D: cosinor analysis of colonic circular muscle contractility. PR, percentage rhythm.
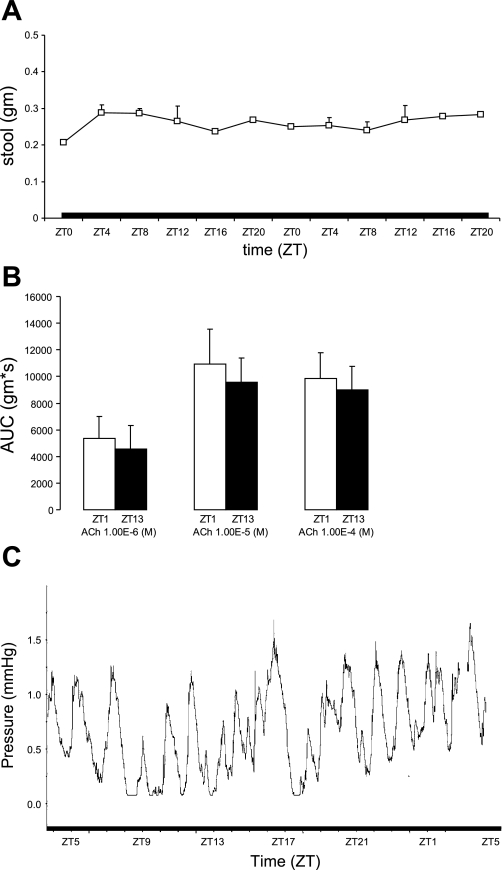
To confirm whether the rhythmic colonic motility was circadian, studies were repeated in constant darkness (DD). Stool output in WT mice remained rhythmic (Fig. 2A), with most of the stool output occurring during the subjective night (Fig. 2B). Colonic circular muscle contractility also was greater at the beginning of the original dark cycle (ZT13) compared with the beginning of the original light phase (ZT1) at all doses of acetylcholine (Fig. 2C). In addition, in vivo intracolonic pressure changes were greatest during the dark phase with ZT0–12 vs. ZT12–0: 1.9 ± 0.1 vs. 3.2 ± 0.3 mmHg/12 h, n = 3 mice, P = 0.0009 (data not shown). A representative tracing of in vivo intracolonic pressure changes in a single WT mouse is shown in Fig. 2D.
Fig. 2.
Rhythmicity in measures of colonic motility in WT mice persists in constant darkness. A: stool output in WT mice (n = 3). B: total stool output during light (ZT0–12) and dark cycle (ZT12–0). C: colonic circular muscle contractility in WT mice at ZT1 (n = 4) and ZT13 (n = 4) expressed as AUC (g·s) ± SE. D: intracolonic pressure changes in a single WT mouse under constant darkness.
Measures of Colonic Motility in per1per2 Double- and per1, per2 Single-Knockout Mice With Ad Libitum access to Food Under Constant Darkness
In contrast to WT mice, per1per2 double-knockout mice did not demonstrate any rhythmicity in stool output (Fig. 3A). The amount of stool weight passed during ZT0–12 did not differ from the amount of stool passed during ZT12–0 (stool output: ZT0–12 vs. ZT12–0: 0.80 ± 0.007 vs. 0.76 ± 0.01 g/12 h, P = not significant, data not shown).
Fig. 3.
Rhythmic changes in colonic motility are attenuated in per1per2 knockout mice. A: stool output in per1per2 double-knockout mice (n = 5). B: colonic circular muscle contractility in per1per2 double-knockout mice at ZT1 (n = 4) and ZT13 (n = 4) expressed as AUC (g·s) ± SE. C: intracolonic pressure changes in per1per2 double-knockout mouse under constant darkness.
Similarly, colonic circular muscle response to acetylcholine did not differ between ZT13 (early subjective night) and ZT1 (early subjective day) in per1per2 double-knockout mice either (Fig. 3B). In addition, there was no difference in in vivo intracolonic pressure changes between ZT0–12 and ZT12–0 in per1per2 double-knockout mice with ZT0–12 vs. ZT12–0: 2.1 ± 0.15 vs. 2.4 ± 0.22 mmHg/12 h, n = 3 mice, P = not significant (data not shown). A representative tracing of in vivo intracolonic pressure changes in a single per1per2 double-knockout mouse is shown in Fig. 2D.
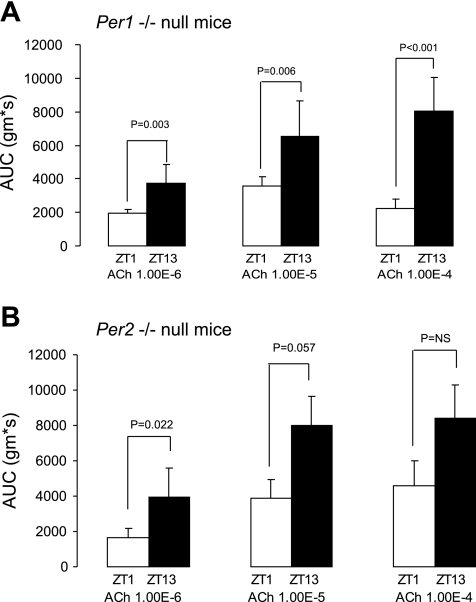
To determine the role of the individual per clock genes, organ bath studies were completed in single-knockout mice as well. Per1 single-knockout mice have a greater contractile response at ZT13 compared with ZT1 (Fig. 4A). Similarly, per2 single-knockout mice expressed greater contractile response at ZT13 compared with ZT1. This difference was of borderline statistical significance for acetylcholine 10−5 and did not achieve statistical significance for acetylcholine 10−4 (Fig. 4B).
Fig. 4.
Colonic circular muscle contractility in per1 and per2 knockout mice. Colonic circular muscle contractility in per1 single-knockout mice (A) and per2 single-knockout mice (B) at ZT1 (n = 4) and ZT13 (n = 4) expressed as AUC (g·s) ± SE. NS, not significant.
Rhythmic Changes in the Colonic Circular Muscle Contractile Response Are Neuronally Mediated
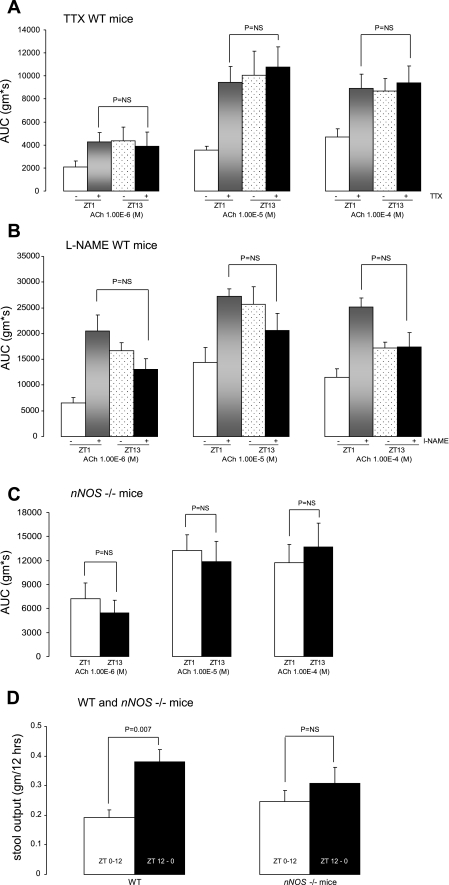
To determine the effect of neural modulation in the rhythmic response of colonic circular muscle contractility to acetylcholine, organ bath studies were repeated in the presence of the neurotoxin TTX, which abolishes neuronal activity. Colonic circular muscle contractility in WT mice does not differ between ZT13 and ZT1 when various doses of acetylcholine are administered in the presence of TTX (Fig. 5A). This is because TTX increased the contractile response at ZT1, indicative of abolishment of inhibitory neuronal activity. In contrast, TTX was without effect at ZT13, presumably because inhibitory neuronal activity is not ongoing in this period.
Fig. 5.
Rhythmic changes in colonic contractile response are neuronally mediated. Colonic circular muscle contractility in WT mice in the absence (−) (n = 4) and presence (+) of TTX (n = 6) (A), in the absence (−) (n = 4) and presence (+) (n = 4) of Nω-nitro-l-arginine methyl ester (l-NAME; B), in nNOS knockout mice (n = 6) (C), and total stool output during ZT0–12 and ZT12–0 in WT and nNOS knockout mice (n = 3) (D).
nNOS Modulates the Inhibitory Response Mediating Rhythmic Changes in Colonic Motility
The enhanced contractile response of the colonic circular muscle at ZT1 in the presence of TTX suggests that TTX abrogated a neuronally mediated inhibitory response at ZT1. To examine the mechanism underlying this inhibitory response, organ bath studies were repeated in the presence of the nNOS inhibitor l-NAME. Colonic circular muscle contractility in WT mice does not differ between ZT13 and ZT1 when various doses of acetylcholine are administered in the presence of l-NAME (Fig. 5B).
To more definitively determine the role of nNOS in the modulation of temporal changes in the circular muscle contractile response to acetylcholine, organ bath studies were repeated in mice genetically deficient in nNOS. Following placement in constant darkness, the colonic circular muscle contractile response in response to acetylcholine in nNOS knockout mice did not differ between the beginning of the light cycle and the beginning of the dark cycle (Fig. 5C). These findings were similar as seen in the presence of TTX.
To determine whether these in vitro findings corroborated with a loss of rhythmicity in stool output, stool studies were completed in WT and nNOS knockout mice in constant darkness. Whereas the stool output in WT continued to be greater during ZT12–0, there was no statistically significant difference in stool output between ZT0–12 and ZT12–0 in nNOS knockout mice (Fig. 5D).
DISCUSSION
Stool output in WT mice (nocturnal animals that are mostly active during the night) is rhythmic with increased output coinciding with the onset of the dark cycle in LD and subjective night in DD. Similarly, the colonic circular muscle contractile response to acetylcholine was also rhythmic in LD and DD. Persistence of rhythmicity in stool output, tissue contractility, and intracolonic pressure changes in DD confirmed that colonic motility is indeed circadian and controlled by an endogenous clock-driven process.
Our data demonstrate that rhythmic changes in the colonic circular smooth muscle contractile response to acetylcholine persist in vitro. These findings suggest that peripheral rhythmicity persists even in the absence of a direct influence of the central clock in the SCN. The ability of peripheral tissues to maintain rhythmicity independent of the central clock is supported by previous observations. First, following subjection to restricted daytime feeding, the colonic clock will shift expression patterns independent of the central clock (11). Second, peripheral clock gene expression can be entrained in mice in which the central clock is ablated (8). Third, Yamazaki et al. (28) demonstrated that explants of the suprachiasmatic nucleus of the brain (containing the central clock) and peripheral tissues (liver, lung, skeletal muscle) from a transgenic Per1 luciferase rat continued to oscillate in vitro. Moreover, the phase of the individual explants paralleled that of the phase in vivo (suggesting that the initial phase in vitro can be used for estimation of the in vivo phase).
Although rhythmicity in per1 single-knockout and per2 single-knockout mice persisted, rhythmicity in the colonic circular muscle response to acetylcholine was abolished in per1per2 double-knockout mice. This is not surprising since other studies have shown that PER proteins are functionally redundant with per1per2 double-knockout mice displaying a stronger phenotype (2). For example, double-knockout mice display an immediate loss of wheel-running rhythmicity when placed in constant darkness as opposed to single-knockout mice. Per1per2 double-knockout mice eat the same amount of food over a 24 h period as WT mice (data not shown). The pattern of food intake is rhythmic in LD (with the greatest amount of food being eaten at night) but attenuated compared with WT mice. This finding is in line with observations made by Yang et al. (29) in per2 knockout mice and suggests that per genes may contribute to phenotypic differences in food intake.
Basal release of inhibitory neurotransmitters keeps smooth muscle cells in the resting state. Muscle contractions occur only when the inhibitory neurons are “switched off” (27). This is based on the following observations: 1) blockade of sodium channels by the neurotoxin TTX (which inhibits the release of neurotransmitters) stimulates muscle contractions (13, 26) and 2) blockade of nNOS by l-NAME enhances acetylcholine-evoked smooth muscle contraction (1, 15, 25). We therefore first explored the role of neural mediation by determining the effect of acetylcholine on colonic circular muscle contractility of WT mice in the presence of the neurotoxin TTX. The previously noted differences in the contractile response to acetylcholine were lost because the response at ZT1 (the beginning of the subjective day) is much greater in the presence of TTX. This suggested that TTX abrogated an inhibitory response that attenuates the response to acetylcholine under normal conditions. We therefore concluded that rhythmic changes in the colonic circular muscle contractile response to acetylcholine are modulated by a neuronally mediated inhibitory mechanism.
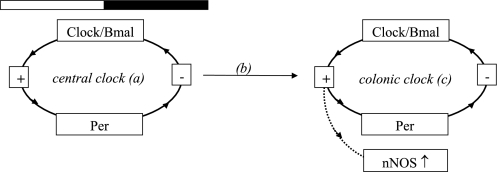
Since previous studies suggested that nNOS, the main inhibitory neurotransmitter in the gastrointestinal tract, is rhythmically expressed in the mouse colon (12), we next determined the effect of acetylcholine on the colonic circular muscle response in the presence of the nNOS inhibitor l-NAME. In the presence of l-NAME there was no longer a difference in the contractile response at ZT1 compared with the contractile response at ZT13. This difference was lost because the response at the beginning of subjective day was much greater in the presence of l-NAME, suggesting that l-NAME abrogated an inhibitory response that attenuated the response to acetylcholine under normal conditions. These findings suggested that the rhythmic changes in the colonic circular muscle response are mediated through a nNOS-dependent mechanism. To confirm these findings, circular muscle contractility to acetylcholine was measured in nNOS knockout mice. nNOS knockout mice are known to have delayed gastric emptying but little is known about the rhythmicity of measures of gastrointestinal motility over the time of day (17). We found that both rhythmic changes in the colonic circular muscle response to acetylcholine as well as stool output were attenuated in nNOS knockout mice. These observations further solidified the role of nNOS in the modulation of rhythmic changes in measures of colonic motility over the time of day. The mechanism through which the period genes may modulate a nNOS-mediated inhibitory effect is summarized in Fig. 6.
Fig. 6.
Proposed paradigm for clock gene-mediated inhibitory effects on colonic motility. Clock genes within the central clock in the brain (a) and within peripheral tissues (c) participate in a complex feedback loop mechanism of cyclical transcription and translation over the course of 24 h [a simplified schematic is presented; detailed review by Bell-Pedersen et al. (3)]. The central clock in the brain synchronizes itself to the light-dark and dictates the phase of peripheral clock genes. The mechanism through which this occurs remains unknown (b). Peripheral clock gene expression lags ∼6–8 h behind the phase of the central clock. Peripheral clock genes will initiate transcription of organ-specific genes through the binding of the CLOCK-Bmal heterodimer complex to a promoter region (direct clock-controlled transcription), which can in turn initiate the transcription of another gene (indirect clock-controlled transcription). It is unknown whether colonic nNOS is under direct or indirect colonic clock control (as indicated by the dotted arrow), but its rhythmic expression mediates an inhibitory effect on colonic motility that coincides with the start of the light cycle.
It should be noted that the overall magnitude of the contractile response to acetylcholine was greater in the presence of l-NAME, and it was also greater overall in nNOS knockout mice compared with WT controls. This increased contractile response in the presence of l-NAME is consistent with observations by others (1, 15, 25) and indicates that inhibition of nNOS, through blockage of the synthesis of the inhibitory neurotransmitter nitric oxide, increases cholinergic contractions. However, the precise mechanism through which inhibition of nNOS increases the contractile response remains to be defined.
Within the gastrointestinal tract, a variety of physiological functions are rhythmic. For example, DNA synthesis and mitosis within epithelial cells, sucrase and maltase activity within the small intestine, and SGLT-1 (a glucose transporter) expression display circadian variation [for a detailed review on circadian rhythms within the gastrointestinal tract, we refer to Scheving et al. (21)]. The identification of the molecular network driving these circadian rhythms has created an opportunity to improve our understanding of the role of circadian rhythms in health and disease. To date, most studies have been completed in rodents. For example, mutations in the clock gene clock have been associated with the development of the metabolic syndrome in mice (24). However, knowledge of the role of clock genes in human health and disease remains limited (10). The only human clock gene mutation known to cause a specific disease is that of a per2 mutation associated with advanced sleep phase syndrome (14). Nonetheless, a close association between disruption of biological rhythms (following participation in shift work) and the development of cardiovascular disease, cancer (including colon cancer), and gastrointestinal symptoms such as constipation or diarrhea has been well established (4, 20, 23). Although extrapolation of our findings to humans should be made with caution, we speculate that clock genes control rhythmic changes in colonic motility over the time of day and that disruption of the molecular clock will lead to alterations in colonic motility and the development of gastrointestinal symptoms. Most recently, it was described that individuals with genetic variations in the clock gene have an increased susceptibility to nonalcoholic liver disease (23). It is conceivable that polymorphisms will play a role in an individual's predisposition to the development of gastrointestinal dysmotility as well. Future studies should elucidate the mechanism through which clock genes regulate colonic motility and explore the role of the molecular clock in the pathogenesis of colonic dysmotility.
GRANTS
This work is supported by R21 DK074477 and the University of Michigan Gastrointestinal Peptide Research Program (W. A. Hoogerwerf).
FINANCIAL DISCLOSURES
None of the authors has a conflict of interest to disclose.
ACKNOWLEDGMENTS
The authors thank Barbara Earnest for breeding and maintenance of per1, per2, and per1per2 knockout mice and Dr. David Weaver of the University of Massachusetts for supplying the original colony.
REFERENCES
- 1.Aube AC, Blottiere HM, Scarpignato C, Cherbut C, Roze C, Galmiche JP. Inhibition of acetylcholine induced intestinal motility by interleukin 1 beta in the rat. Gut 39: 470–474, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 30: 525–536, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet 6: 544–556, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caruso CC, Lusk SL, Gillespie BW. Relationship of work schedules to gastrointestinal diagnoses, symptoms, and medication use in auto factory workers. Am J Ind Med 46: 586–598, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Cassone VM, Stephan FK. Central and peripheral regulation of feeding and nutrition by the mammalian circadian clock: implications for nutrition during manned space flight. Nutrition 18: 814–819, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Costa G. The impact of shift and night work on health. Appl Ergon 27: 9–16, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Gangula PR, Maner WL, Micci MA, Garfield RE, Pasricha PJ. Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. Am J Physiol Gastrointest Liver Physiol 292: G725–G733, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, Shibata S. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells 6: 269–278, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Hoogerwerf WA. Biologic clocks and the gut. Curr Gastroenterol Rep 8: 353–359, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Hoogerwerf WA. Role of biological rhythms in gastrointestinal health and disease. Rev Endocr Metab Disord doi: 10.1007/sl1154-009-9119-3 [DOI] [PubMed] [Google Scholar]
- 11.Hoogerwerf WA, Hellmich HL, Cornelissen G, Halberg F, Shahinian VB, Bostwick J, Savidge TC, Cassone VM. Clock gene expression in the murine gastrointestinal tract: endogenous rhythmicity and effects of a feeding regimen. Gastroenterology 133: 1250–1260, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Hoogerwerf WA, Sinha M, Conesa A, Luxon BA, Shahinian VB, Cornelissen G, Halberg F, Bostwick J, Timm J, Cassone VM. Transcriptional profiling of mRNA expression in the mouse distal colon. Gastroenterology 135: 2019–2029, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou JY, Otterson MF, Sarna SK. Local effect of substance P on colonic motor activity in different experimental states. Am J Physiol Gastrointest Liver Physiol 256: G997–G1004, 1989 [DOI] [PubMed] [Google Scholar]
- 14.Jones CR, Campbell SS, Zone SE, Cooper F, DeSano A, Murphy PJ, Jones B, Czajkowski L, Ptacek LJ. Familial advanced sleep-phase syndrome: a short-period circadian rhythm variant in humans. Nat Med 5: 1062–1065, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Knudsen MA, Tottrup A. A possible role of the l-arginine-nitric oxide pathway in the modulation of cholinergic transmission in the guinea-pig taenia coli. Br J Pharmacol 107: 837–841, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knutsson A. Health disorders of shift workers. Occup Med (Lond) 53: 103–108, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Mashimo H, Kjellin A, Goyal RK. Gastric stasis in neuronal nitric oxide synthase-deficient knockout mice. Gastroenterology 119: 766–773, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Moore RY, Silver R. Suprachiasmatic nucleus organization. Chronobiol Int 15: 475–487, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Rao SS, Sadeghi P, Beaty J, Kavlock R, Ackerson K. Ambulatory 24-h colonic manometry in healthy humans. Am J Physiol Gastrointest Liver Physiol 280: G629–G639, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Fuchs CS, Colditz GA. Night-shift work and risk of colorectal cancer in the nurses' health study. J Natl Cancer Inst 95: 825–828, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Scheving LA. Biological clocks and the digestive system. Gastroenterology 119: 536–549, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Sladek M, Rybova M, Jindrakova Z, Zemanova Z, Polidarova L, Mrnka L, O'Neill J, Pacha J, Sumova A. Insight into the circadian clock within rat colonic epithelial cells. Gastroenterology 133: 1240–1249, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Sookoian S, Castano G, Gemma C, Gianotti TF, Pirola CJ. Common genetic variations in CLOCK transcription factor are associated with nonalcoholic fatty liver disease. World J Gastroenterol 13: 4242–4248, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308: 1043–1045, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiklund CU, Wiklund NP, Gustafsson LE. Modulation of neuroeffector transmission by endogenous nitric oxide: a role for acetylcholine receptor-activated nitric oxide formation, as indicated by measurements of nitric oxide/nitrite release. Eur J Pharmacol 240: 235–242, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Wood JD. Excitation of intestinal muscle by atropine, tetrodotoxin, and xylocaine. Am J Physiol 222: 118–125, 1972 [DOI] [PubMed] [Google Scholar]
- 27.Wood JD, Alpers DH, Andrews PL. Fundamentals of neurogastroenterology. Gut 45, Suppl 2: II6–II16, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science 288: 682–685, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Yang S, Liu A, Weidenhammer A, Cooksey RC, McClain D, Kim MK, Aguilera G, Abel ED, Chung JH. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology 150: 2153–2160, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]