Figure 3.
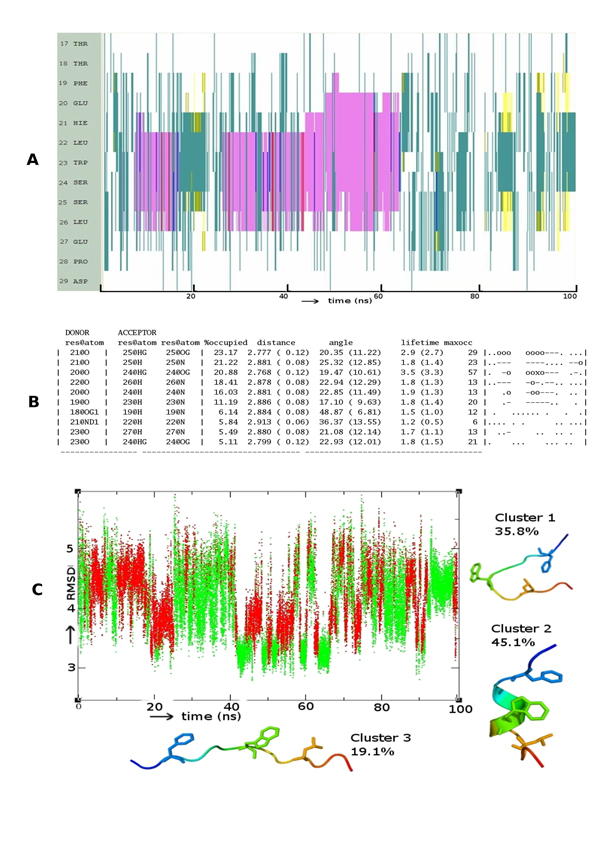
Folding pattern of p73 Evolution of secondary structures of the p73 peptides as a function of simulation time Colour code: purple, α-helix; red, π-helix; yellow, β-sheet; green, isolated bridge; cyan, turn; white, random coil. (B) Hydrogen bond statistics of the secondary structures averaged over 100 ns of simulations; the lifetime of hydrogen bonds in 5 ns windows is shown as: Space ( ) for 0-5%, dot (.) for 5-20%, dash (-) for 20-40%, o for 40-60%, x for 60-80%, star (*) for 80-95% and at (@) for 95 – 100%. (C) Cluster analysis of secondary structures in terms of RMSD as a function of simulation time; a representative structure (N-terminus in blue, C-terminus in red) from each cluster is shown with % of population; colour code of the plot: red is helix, yellow is β-Sheet and green is random structure. Conserved residues F19, W23 and L26 are shown as sticks.
