Table 1.
Coupling of Heteroaryl Halides with Primary Alkylamines Catalyzed by Pd(OAc)2 and CyPF-t-Bu (1:1).a
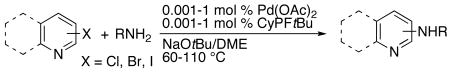 | ||||||
|---|---|---|---|---|---|---|
| Entry | Ar | X | R | Cat. (%) |
Conditions | Yield (%)b |
| 1 | 2-Py | Cl | Octyl | 0.001 | 100 °C, 48 h | 86 |
| 2 | Br | Octyl | 0.0005 | 110 °C, 12 h | 84 | |
| 3 | I | Octyl | 0.005 | 110 °C, 12 h | 96 | |
| 4 | Cl | Bn | 0.001 | 100 °C, 10 h | 85 | |
| 5 | Br | Bn | 0.0005 | 110 °C, 12 h | 83 | |
| 6 | Cl | Cyclohexyl | 0.01 | 70 °C, 12 h | 98 | |
| 7 | Br | Cyclohexyl | 0.005 | 60 °C, 12 h | 96 | |
| 8 | I | sec-Bu | 0.05 | 110 °C, 12 h | 98 | |
| 9d | Cl | 1-Methylbenzylf | 0.05 | 100 °C, 24 h | 99 | |
| 10 | 3-Me-2-Py | Cl | Octyl | 0.005 | 90 °C, 24 h | 96 |
| 11 | 3-Py | Cl | Octyl | 0.005 | 90 °C, 24 h | 93 |
| 12c | Cl | Octyl | 0.005 | 100 °C, 24 h | 99 | |
| 13 | Br | Octyl | 0.005 | 100 °C, 36 h | 99 | |
| 14 | Cl | Bn | 0.01 | 100 °C, 24 h | 95 | |
| 15 | Br | Bn | 0.005 | 100 °C, 48 h | 99 | |
| 16 | Br | secBu | 0.005 | 100 °C, 48 h | 93 | |
| 17 | I | isoBu | 0.05 | 100 °C, 12 h | 96 | |
| 18 | Cl | Cyclohexyl | 0.01 | 100 °C, 48 h | 79 | |
| 19 | I | Cyclohexyl | 0.05 | 100 °C, 12 h | 78 | |
| 20 | Cl | tert-Bu | 1.0 | 70 °C, 16 h | 67 | |
| 21d | Cl | 1-Methylbenzylg | 0.05 | 100 °C, 24 h | 91 | |
| 22 | 4-Py | Cl | Octyl | 0.01 | 90 °C, 24 h | 83 |
| 23 | Br | Octyl | 0.005 | 100 °C, 3 6h | 93 | |
| 24 | I | Octyl | 0.05 | 100 °C, 48 h | 80 | |
| 25 | Cl | Phenylcarbonyl | 1.0 | 70 °C, 10 h | 75 | |
| 26 | 2-Pyrazinyl | Cl | Octyl | 0.005 | 100 °C, 16 h | 82 |
| 27 | I | Octyl | 0.05 | 100 °C, 36 h | 83 | |
| 28e | 1,3-Pyrimidyl-5- | Br | Cyclohexyl | 1.0 | 100 °C, 48 h | 80 |
| 29 | 3-Quinolinyl | Cl | Cyclohexyl | 0.01 | 100 °C, 48 h | 60 |
| 30 | 1-isoQuinolinyl | Cl | Octyl | 0.005 | 90 °C, 15 h | 91 |
| 31 | 4-isoQuinolinyl | Br | Octyl | 0.005 | 100 °C, 36 h | 93 |
Reactions conducted with a 1:1 ratio of metal to ligand, 1 mmol aryl halide, 1.2 equiv amine, and 1.4 equiv NaOtBu in 1 mL DME.
Isolated yield;
Reaction performed without using a drybox.
from phenethylamine that is stated to be 99% ee.
Using K3PO4 as the base.
96% ee.
95% ee.
