Table 4.
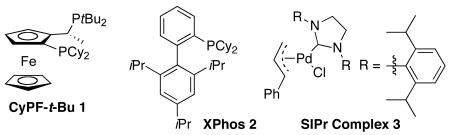
Comparison of the Activity of CyPF-t-Bu, XPhos, and SiPr for the Reactions of 4-Chlorotoluene with a Primary Alkylamine.a
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Ligand | Loading | Solvent | T[°C]b | t [ h] | Conversionc (%) |
A:B |
| 1 | Pd(OAc)2/1 | 0.01 | DME | 100 | 48 | 100(95) | 100:0 |
| 2 | Pd(OAc)2/2 | 0.05 | Toluene | 100 | 48 | 13 | 18:1 |
| 3 | Pd(dba)2/2 | 0.1 | Toluene | 100 | 48 | 25 | 10:1 |
| 4 | Pd(OAc)2/2 | 0.1 | Toluene | 100 | 48 | 16 | 39:1 |
| 5 | Pd(OAc)2/2 | 0.5 | Toluene | 100 | 12 | 96(91) | 2.7:1 |
| 6 | Pd(dba)2/2 | 0.5 | Toluene | 100 | 12 | 100(93) | 2.3:1 |
| 7 | 3 | 0.005 | Toluene | 110 | 48 | 40 | >50:1 |
| 8 | 3 | 0.05 | Toluene | 110 | 48 | 76 | 15.3:1 |
| 9 | 3 | 0.5 | Toluene | 110 | 12 | 100 | 4.3:1 |
 | |||||||
The experiments were conducted with a 1:1 ratio of Pd/CyFP-t-Bu or 1:2 ratio of Pd/XPhos, 1 mmol of 4-chlorotoluene and 1.2 equiv 1-octylamine, and 1.4 equiv base in 1.0 mL solvent.
Bath temperature.
Determined by 1H NMR analysis of the crude product. Isolated yields are indicated in parentheses.
