Table 9.
Coupling of Functionalized Aryl Halides with Primary Amines Catalyzed by Pd(OAc)2 and CyPF-t-Bu (1:1).a
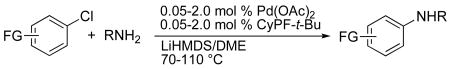 | ||||||
|---|---|---|---|---|---|---|
| Entry | -FG | X | R | Cat. (%) |
Conditions | Yieldb (%) |
| 1 | -4-COOH | Cl | Octyl | 0.05 | 100 °C, 20 h | 81 |
| 2 | Br | Octyl | 0.05 | 100 °C, 20 h | 72 | |
| 3 | Br | secBu | 0.05 | 100 °C, 20 h | 92 | |
| 4 | I | secBu | 0.05 | 100 °C, 48 h | 78 | |
| 5 | -4-CONH2 | Cl | isoBu | 0.5 | 100 °C, 20 h | 67 |
| 6 | Br | isoBu | 0.05 | 100 °C, 20 h | 85 | |
| 7c | -4-COCH3 | Cl | Octyl | 0.5 | 70 °C, 20 h | 91 |
| 8d | Cl | Octyl | 0.5 | 110 °C, 24 h | 74 | |
| 9 | Cl | Octyl | 0.05 | 100 °C, 16 h | 61 | |
| 10d | I | Octyl | 1.0 | 110 °C, 24 h | 78 | |
| 11c | Cl | Cyclohexyl | 0.5 | 70 °C, 20 h | 87 | |
| 12d | Br | Cyclohexyl | 1.0 | 110 °C, 24 h | 82 | |
| 13 | -4-NHCOCH3 | Br | Octyl | 0.05 | 100 °C, 20 h | 98 |
| 14 | I | Octyl | 0.5 | 110 °C, 20 h | 67 | |
| 15 | -3-NHCOCH3 | Cl | Cyclohexyl | 0.05 | 100 °C, 24 h | 74 |
| 16 | -4-CH2OH | Br | isoBu | 0.05 | 100 °C, 48 h | 72 |
| 17 | I | isoBu | 0.05 | 100 °C, 20 h | 47 | |
| 18 | -4-CH(OH)CH3 | Cl | secBu | 0.5 | 100 °C, 18 h | 83 |
| 19 | -4-CH2CH2OH | Cl | Octyl | 0.5 | 100 °C, 20 h | 89 |
| 20 | -3-OH | Cl | Bn | 0.5 | 100 °C, 20 h | 84 |
| 21 | Br | Bn | 0.5 | 100 °C, 20 h | 85 | |
| 22 | Br | Cyclohexyl | 0.5 | 100 °C, 36 h | 81 | |
| 23 | I | Cyclohexyl | 1.0 | 100 °C, 20 h | 70 | |
| 24 | -4-OH | Cl | Octyl | 2.0 | 100 °C, 18 h | 72 |
| 25d | -4-CO2Me | Cl | isoBu | 1.0 | 110 °C, 24 h | 94 |
| 26d | -4-CO2Et | I | secBu | 2.0 | 110 °C, 48 h | 77 |
| 27d | -3-CO2Me | Cl | isoBu | 1.0 | 110 °C, 24 h | 91 |
| 28d | Br | isoBu | 1.0 | 110 °C, 24 h | 97 | |
| 29d | -2-CO2Me | Cl | Bn | 2.0 | 110 °C, 24 h | 82 |
| 30d | -4-NO2 | I | Octyl | 1.0 | 110 °C, 24 h | 79 |
Reactions conducted with a 1:1 ratio of metal to ligand, 1.0 mmol ArX (X = Cl, Br, I), 1.2 equiv amine, and 2.4 equiv LiN(SiMe3)2 in 1 mL DME.
Isolated yields.
Reactions run at 0.2 M.
Using K3PO4 as the base.
