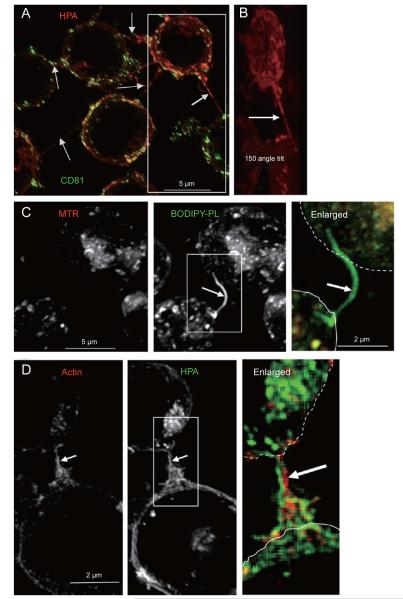
Figure 2.
Fas signalling increases the formation of connecting membrane nanotubes. (A) The image shows the cumulative z-projection of 29 sections of cells, which were surface stained with red HPA after internalization of FITC-conjugated anti-CD81 as described earlier [13]. Arrows point to the various connecting nanotubes that emerge at different equatorial levels along the z axis. Deconvolved images were taken with a × 100 objective. (B) The image is slightly enlarged from the box area in A and tilted by an angle of 150 degrees to better show the long connecting nanotube sprouting from the central part of the top cell. Only the surface red staining is shown. (C) Cells were incubated with 50 nM Mitotracker® red (MTR) and then with liposomes containing fluorescent BODIPY-phosphatidylcholine (Molecular Probes) plus BODIPY-monolysocardiolipin (Degli Esposti, unpublished) mixed with non-fluorescent membrane phospholipids. After equilibration and washing, fluorescent lipids were predominantly endocytosed and accumulated in nanotubular protrusions (arrow), similar to carbocyanine lipophilic dyes (cf. [21]). (D) After 30 min of treatment with the Fas-activating antibody CH-11, Jurkat cells were fixed, permeabilized with 0.2% saponin and then incubated with 10 μM Texas red-conjugated phalloidin (red, to stain F-actin) and then stained with 2 μg/ml of AlexaFluor488-conjugated HPA (green). Arrow indicates the large connecting nanotube enriched in actin (enlarged).