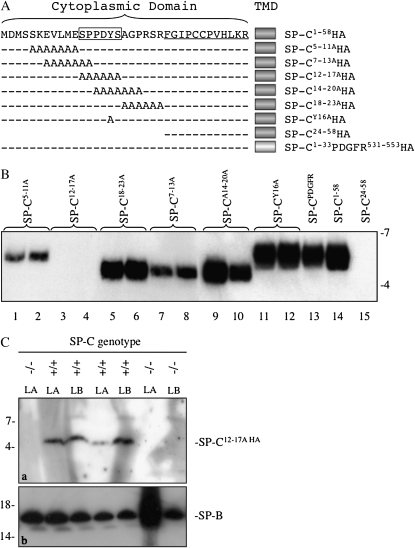
Figure 1.
Identification of a motif required for secretion of surfactant protein (SP)–C by type II epithelial cells. (A) Alanine mutagenesis of the NH2-terminal propeptide of SP-C was performed, and adenovirus encoding the mutated cytosolic domain, native transmembrane domain (TMD), and a C-terminal hemagglutinin (HA) tag was generated for each construct. Underlined residues constitute the cytosolic domain of the mature peptide; boxed residues include a PY motif (PPDY). (B) Mouse type II cells were isolated from Sftpc−/− mice, cultured for 2 days, then infected in duplicate with adenovirus encoding wild-type (WT) SP-C (SP-C1–58HA), SP-C carrying alanine substitutions in the propeptide (SP-CnHA), SP-C in which the TMD was replaced with the comparable domain from PDGFR (SP-C1–33 PDGFR531–553HA), or mature SP-C peptide (SP-C24–58HA). Media were collected 24 hours later, the surfactant pellet isolated by centrifugation, and secreted SP-C detected by SDS-PAGE/Western blotting with HA antibody. Mature SP-CHA migrated with relative electrophoretic mobility (Mr) of approximately 5 k or approximately 6 k. Representative of two experiments with each construct analyzed in duplicate. (C) WT (+/+) or Sftpc (−/−) mice were intratracheally infected with adenovirus encoding SP-C12–17AHA. At 3 days after infection, mice were lavaged and the large aggregate (LA) fraction of surfactant recovered; lamellar bodies (LBs) were isolated from lung homogenates by flotation on sucrose gradients. SP-C was detected by SDS-PAGE/Western blotting with HA antibody. (a) Mature SP-C12–17AHA, Mr approximately 5 k. (b) LA and LB fractions were immunoblotted for mature SP-B; Mr, approximately 16 k. Representative of two experiments (see Figure S1B).