Figure 4.
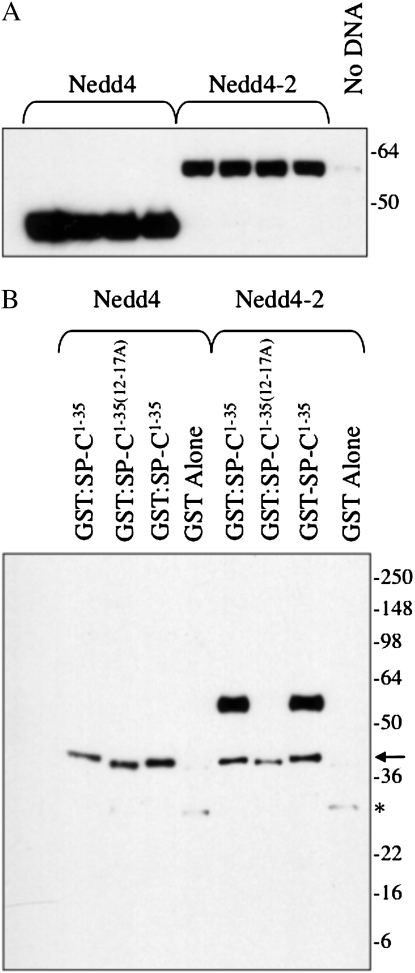
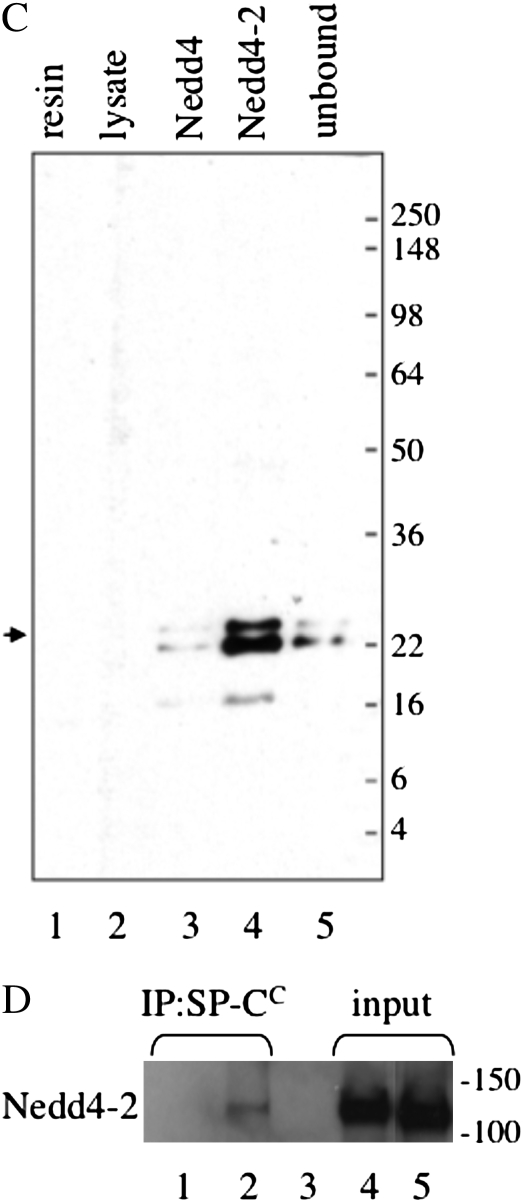
Neural precursor cell–expressed developmentally down-regulated (Nedd) 4-2 binds to the cytosolic domain of proSP-C. (A) Plasmids encoding the WW domains of mouse Nedd4 or Nedd4-2 were transfected into HEK293 cells. At 48 hours after transfection, cell lysates were prepared and analyzed by SDS-PAGE/Western blotting with antibody directed against the Nedd4 WW domain. (B) Cell lysates (seen in [A]) were incubated with purified, recombinant GST–SP-C1–35 encoding the cytosolic domain of WT proSP-C or GST–SP-C1–35(12–17A) in which the SPPDYS motif was mutated to alanine. Complexes were captured with glutathione agarose and analyzed by SDS-PAGE/Western blotting with WW domain antibody. Arrow and asterisk indicate cross-reactivity of antibody with GST–SP-C and GST, respectively. (C) Recombinant Nedd4 or Nedd4-2 domain was captured from transfected HEK293 cells (seen in [A]) with HA antibody conjugated to protein G sepharose. Freshly isolated mouse type II epithelial cells were lysed and serially incubated with protein G sepharose (lysate), Nedd4, and Nedd4-2. The unbound type II cell lysate (lane 5) and protein G sepharose complexes (lanes 1–4) were subsequently analyzed by SDS-PAGE/Western blotting with antibody directed against the N-terminal peptide of proSP-C. Small arrow indicates proSP-C. (D) Type II cells isolated from WT (lanes 2, 3, and 5) or Sftpc−/− mice (lanes 1 and 4) were lysed and immunoprecipitated with antibody directed against the C-terminal, lumenal domain of SP-C (lanes 1 and 2). Captured proSP-C complexes were analyzed by SDS-PAGE, followed by Western blotting with WW domain antibody (lanes 1 and 2). Lane 1, type II cell lysate from Sftpc−/− mice; lane 2, type II cell lysate from WT mice; lane 3, type II cell lysate from WT mice immunoprecipitated with protein G sepharose (no SP-C antibody); lane 4, input lysate from Sftpc−/− type II cells; lane 5, input lysate from WT type II cells.