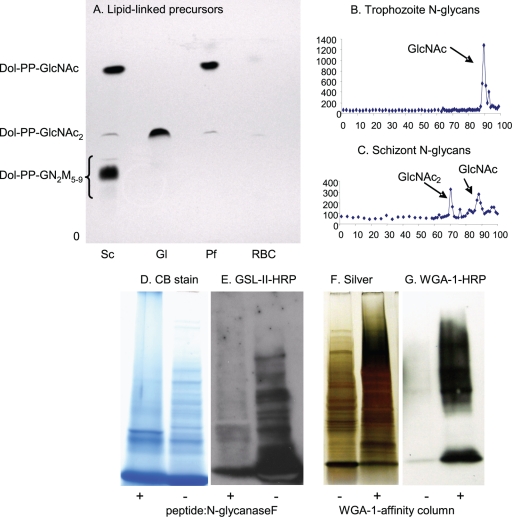
Fig. 3.
Plasmodia metabolically labeled with tritiated glucosamine (GlcN) make N-glycan precursors and N-glycans composed of a single GlcNAc and of GlcNAc2. (A) TLC shows that Plasmodium (Pf) trophozoites (early stages) make predominantly dolichol-PP-GlcNAc with a slight amount of dolichol-PP-GlcNAc2. For comparison, Giardia (Gl), which has the same Alg enzymes as Plasmodium (Fig. 1) (46), makes predominantly dolichol-PP-GlcNAc2, while Saccharomyces (Sc) makes a mixture of dolichol-PP-GlcNAc2 and high-mannose dolichol-PP-glycans. (B) N-glycans released by PNGaseF from glycoproteins of Plasmodium trophozoites are composed predominantly of a single GlcNAc. (C) N-glycans of Plasmodium schizonts (later stages) are a mixture of GlcNAc and GlcNAc2. (D to G) Additional biochemical evidence for short N-glycans of Plasmodium is shown by blots with the GlcNAc-binding lectin GSL-II after treatment of proteins with PNGaseF and by WGA-1 blots of WGA-1-affinity-purified proteins. PNGaseF-treated Plasmodium proteins, which are stained with Coomassie blue (D), show a marked decrease in binding GSL-II (E). This result shows that GSL-II is binding to N-glycans. WGA-1-affinity markedly enriches Plasmodium proteins, which are stained with silver (F), that bind to the lectin (G). Proteins identified by mass spectroscopy after a representative WGA-1 affinity experiment are shown in Table 1.