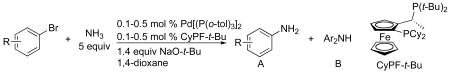
Table 3.
Coupling of ortho-Substituted Aryl Bromides with Ammoniaa
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| entry | aryl bromide | loading (%) | concentration [M] | T [°C]b | t [h] | product | yield (%)c | A:Bd | |
| 1 | 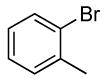 |
0.5 | 0.038 | 80 | 4 | 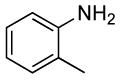 |
83 | >50:1 | |
| 2 | 0.1 | 0.1 | 100 | 12 | 71 | 17:1 | |||
| 3 | 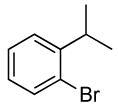 |
0.5 | 0.07 | 80 | 4 | 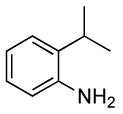 |
93 | >50:1 | |
| 4 | 0.1 | 0.1 | 100 | 12 | 84 | >50:1 | |||
| 5 | 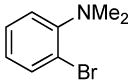 |
0.5 | 0.07 | 80 | 4 | 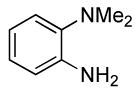 |
89 | >50:1 | |
| 6 | 0.1 | 0.1 | 100 | 12 | 82 | >50:1 | |||
| 7 | 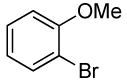 |
0.5 | 0.038 | 80 | 4 | 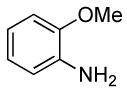 |
85 | >50:1 | |
| 8 | 0.1 | 0.1 | 100 | 12 | 95 | >50:1 | |||
| 9 | 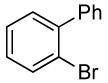 |
0.5 | 0.038 | 80 | 12 | 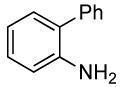 |
96 | >50:1 | |
| 10 | 0.1 | 0.1 | 100 | 24 | 99 | >50:1 | |||
| 11 | 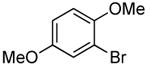 |
0.1 | 0.1 | 100 | 12 |  |
95 | >50:1 | |
| 12 | 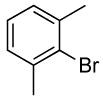 |
0.5 | 0.1 | 80 | 15 | 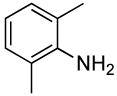 |
88 | >50:1 | |
| 13 | 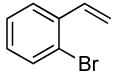 |
0.5 | 0.038 | 80 | 5 | 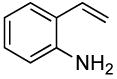 |
66 | -- | |
Reactions conducted with 1:1 ratio of metal to ligand, 0.5 mmol aryl bromide, 5 mL of 0.5 M ammonia solution, 1.4 equiv NaOtBu in 1,4-dioxane.
Temperature of bath.
Isolated yield after purification by flash column chromatography.
Determined by 1H NMR spectroscopy.
