Table 7.
Coupling of Ammonia with Aryl Tosylatesa
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| entry | aryl bromide | loading (%) | concentration [M] | T [°C]b | t [h] | product | yield (%)c | A:Bd |
| 1 | 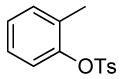 |
2 | 0.1 | 50 | 24 | 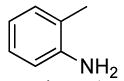 |
65 | 17:1 |
| 2 | 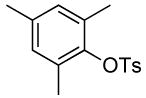 |
2 | 0.1 | 50 | 24 | 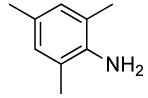 |
86 | >50:1 |
| 3 | 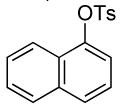 |
2 | 0.05 | 50 | 24 | 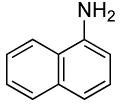 |
67 | >50:1 |
| 4 | 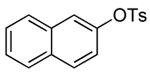 |
2 | 0.05 | 50 | 24 | 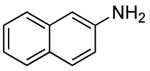 |
67 | --e |
| 5 | 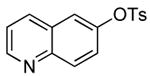 |
2 | 0.05 | 50 | 24 | 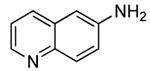 |
55 | --e |
Reactions conducted with 1:1 ratio of metal to ligand, 0.5 mmol aryl chloride, 5 mL of 0.5 M ammonia solution, 1.4 equiv NaO-t-Bu in 1,4-dioxane.
Temperature of bath.
Isolated yield after purification by flash column chromatography.
Determined by 1H NMR spectroscopy.
Selectivity was not determined due to overlapping resonances in the aromatic region of the 1H NMR spectra of the crude reaction mixtures.
