Table 9.
Synthesis of Amides and Imides from Aryl Bromides and Chloridesa
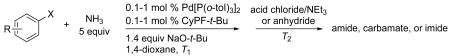 | |||||||
|---|---|---|---|---|---|---|---|
| entry | aryl halide | electrophile | loading (%) | T1 (°C)b | T2 (°C)b | product | yield (%)c |
| 1d |  |
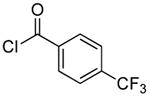 |
0.1 | 100 | 25 | 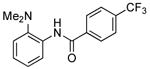 |
89 |
| 2 | 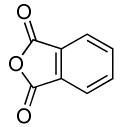 |
0.1 | 100 | 25 |  |
51 | |
| 3d | 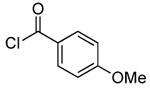 |
0.1 | 100 | 50 | 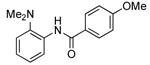 |
74 | |
| 4 | Boc2 | 0.1 | 100 | 80 | 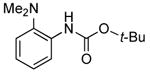 |
75 | |
| 5d | 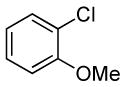 |
 |
0.1 | 100 | 50 | 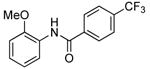 |
82 |
| 6d | 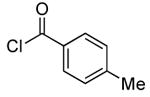 |
0.1 | 100 | 50 | 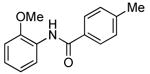 |
94 | |
| 7 | Boc2 | 0.1 | 100 | 80 | 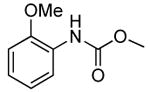 |
68 | |
| 8 | 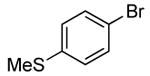 |
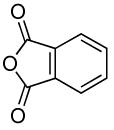 |
0.1 | 90 | 25 | 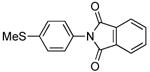 |
75 |
Reactions conducted with 1:1 ratio of metal to ligand.
Temperature of bath.
Isolated yield after purification by flash column chromatography.
1 equiv of Et3N was added.
