Abstract
Many topical pharmaceuticals such as aerosols, topical sprays, and hydro-alcoholic and polymer based gels contain chemical enhancers. The objectives of the present study were to (a) determine the enhancement effects induced by enhancers deposited from a volatile solvent on human epidermal membrane (HEM) upon transdermal permeation enhancement, (b) compare these enhancement factors with Emax, and (c) examine the relationship between enhancer-induced permeation enhancement and stratum corneum equilibrium uptake enhancement. In this study, HEM was treated with enhancer/ethanol (enhancer dissolved in ethanol). After the evaporation of ethanol, passive transport experiments were conducted using corticosterone (CS) as the model permeant. The uptake of another model corticosteroid, estradiol (E2β), into the intercellular lipid domain of stratum corneum after enhancer/ethanol treatment was also determined. The results show a correlation between Emax and the enhancement effect of most enhancers when the enhancers were deposited on the skin using the volatile solvent ethanol. The data suggest that the CS transport rate limiting domain was likely the same as the intercellular lipid domain probed by E2β uptake. The correlation between steady-state permeation enhancement and uptake enhancement into the intercellular lipid domain suggests that the permeation enhancement mechanism is primarily due to enhancement of permeant partitioning into the transport rate limiting domain.
Keywords: Chemical permeation enhancer, transdermal, maximum enhancement effect (Emax), β-estradiol, corticosterone
INTRODUCTION
Transdermal delivery has numerous advantages over other conventional routes of drug administration, yet the skin basic barrier function imposes a limitation to its utility. Many penetration enhancement methods such as physical, chemical, and biological enhancers have been studied to overcome the barrier function of skin. Among these skin permeation enhancement methods, chemical enhancers have been the most widely used. Although a significant number of chemicals have been classified as chemical enhancers, only a limited number of these chemicals are incorporated in topical or transdermal products possibly due to limited knowledge of the mechanism of these enhancers or undesirable biological effects such as skin irritation [1]. A mechanistic study of these enhancers would facilitate proper enhancer selection based on the physicochemical properties of the enhancers rather than extensive and time consuming enhancer screening.
In a previous study, the permeation enhancement induced by an enhancer on the lipoidal pathway of human epidermal membrane (HEM) when an enhancer approached its highest thermodynamic activity in equilibrium with HEM was defined as the maximum intrinsic enhancement effect (Emax) [2]. It was concluded in the study that: (a) a general relationship exists between Emax and octanol-water partition coefficient (Koct) of the enhancers, (b) the enhancer mechanism of action and the interaction between the enhancer and stratum corneum (SC) lipid domain are rather non-specific among the different chemical enhancer groups studied, (c) the maximum enhancer effects depend on the enhancer solubility in the lipid domain of HEM SC, (d) the durations of the enhancement effect induced by the enhancers are related to their lipophilicities, and (e) the solubilities of the enhancers in silicone elastomer could be used as a predictor of Emax.
The presentation of enhancers, in equilibrium with HEM, at their highest thermodynamic activity resembles a number of topical preparations composed of drugs, volatile solvents, and chemical enhancers [3–7]. These topical preparations include gels, topical aerosols, and spray systems that contain volatile solvent(s) such as ethanol and/or water which evaporates upon application leaving the active drug and chemical enhancer on the skin surface. It has been suggested that these delivery systems are more convenient and patient friendly in comparison to other delivery systems such as transdermal patches [8]. For example, the metered dose aerosol system for the systemic delivery of drugs such as testosterone, estradiol, and buspirone has been recently investigated [9]. Estradiol, in particular, was shown to be successfully delivered through skin using the aerosol system and to provide the appropriate systemic dose for postmenopausal women [10]. In order to properly select chemical enhancers that can be used in these systems, the enhancement effect induced solely by the enhancer should be studied. In many other enhancer studies, co-solvents and solubilizing agents that may have an impact on skin integrity were used [11, 12] and possible synergistic effect between enhancer and co-solvent or solubilizing agent is difficult to delineate [13, 14]. The effectiveness of an enhancer determined in these experiments, would only be indicative of an enhancer effect under the experimental conditions stated and may not be extrapolated to other systems such as the hydro-alcoholic gel and aerosol spray products.
Several recent studies have focused on establishing a structure enhancement relationship between chemical permeation enhancers and their enhancement effects [1, 15–18]. The mechanism by which chemical enhancers affect the SC lipoidal domain has been previously investigated using corticosterone (CS) as a model permeant [17, 19–21]. In these studies, the effect of enhancers on the partitioning and uptake of β-Estradiol (E2β) into the intercellular lipid domain of SC from enhancer solution was also determined. It was hypothesized that permeation enhancement was related to permeant partitioning enhancement into the SC lipid domain.
The present study examined the enhancement effects of chemical enhancers deposited on the surface of HEM from a volatile solvent upon transdermal permeation of a lipophilic compound that utilizes the lipoidal pathway. The objectives were to (a) determine the flux enhancement induced by enhancer deposition from a volatile solvent system, (b) examine the relationship between the enhancer enhancement factors and enhancer Emax, (c) determine the uptake enhancement of E2β induced by the enhancers, and (d) identify a possible correlation between the permeant uptake and permeation enhancement effects of the enhancers.
EXPERIMENTAL METHOD
Materials
3H-CS was purchased from Perkin Elmer Life and Analytical Sciences (Boston, MA) at purity > 97%. Micronized E2β USP was purchased from Letco (Decatur, AL) and Spectrum Chemical and Laboratory Supplies (Gardena, CA) at purity ≥ 97%. Isopropyl myristate (IPM) and oleyl alcohol (OA) were purchased from Alfa Aeser (Ward Hill, MA) at purities of 98% and 85% respectively. 2-phenoxyethanol (PHE), n-octanol (OC), 1-undecanol (UD), and sodium azide (NaN3) were purchased at purities ≥98% from Acrōs Organics (Morris Plains, NJ). 1-Octyl-2-pyrrolidinone (OP), N-dodecylpyrrolidinone (DoP), and 2-ethyl hexylsalicylate (OS) were purchased from Sigma-Aldrich Co. (Saint Louis, MO) at purities > 98%. Laurocapram (AZ) was purchased from NETQEM (Durham, NC) at 91% purity. Padimate O (PADO) was purchased from Spectrum Chemicals (Gardena, CA) at purity > 90%. Trypsin from bovine pancreas was purchased from MP biomedical (Santa Ana, CA).
Phosphate buffered saline (PBS: 0.01 M phosphate buffer, 0.0027 M potassium chloride, 0.137 M sodium chloride) pH 7.4, was prepared as described by the manufacturer. 0.02% NaN3 was used as a preservative in PBS. Posterior torso split thickness cadaver skin of 16 donors was obtained from the New York Fire Fighters Skin Bank (New York, NY). Human epidermal membrane (HEM) was prepared by heat separation and then stored in a −20° C freezer for later use [22].
HEM Transport Experiments: Control
HEM samples were cut into squares of 1.2–1.5 cm each side and were allowed to thaw and equilibrate for two hours in 20 mL PBS in a Petri dish at room temperature. HEM was then lifted up with a filter paper support, and the HEM-filter composite was placed on a cotton support wetted with PBS in a Petri dish with SC facing up. 0.35 mL of ethanol was then pipetted on the HEM. The 0.35-mL was selected based on a calculation that this volume of ethanol would evaporate in about one minute [23]. The rapid evaporation was necessary in order to minimize the impact of ethanol on the integrity of HEM and to mimic the condition when a volatile solvent delivery system is used in practice. After treatment, HEM was allowed to equilibrate on the PBS wetted support for at least 20 minutes. HEM was then rinsed three times with PBS and mounted in a side-by-side diffusion cell as described previously [2]. Briefly, HEM was mounted with SC facing the donor chamber between Millipore filter (Durapore membrane filters, 0.22 μm pore diameter) and rubber gaskets one on each side of HEM. 2 mL of PBS were pipetted into both the donor and the receiver chamber and allowed to equilibrate under well stirred conditions at 37° C for two hours in a circulating water bath. After equilibration, PBS in both the receiver and donor chambers were completely replaced with fresh PBS.
The integrity of HEM samples was checked by the electrical resistance of HEM. HEM electrical resistance was determined by passing an electric current through HEM to provide 0.1 V across the membrane, and the resistance was calculated using the current, voltage drop across HEM, and Ohm’s law [2]. HEM samples with resistance ≥15 kΩcm2 were deemed of suitable integrity and were used in the transport study [2]. Electrical resistance was also used to determine the impact of the enhancer treatment on the permeability of the polar pathway of HEM. After the integrity testing, 3H-CS (≈0.1 μCi) was pipetted into the donor chamber. Passive transport experiments were conducted under well-stirred conditions for at least 6 hours, which was about 6 times the transport lag time of CS across HEM. 2 mL and 10 μL samples were taken from the receiver and donor chambers, respectively, at predetermined time intervals. The samples were then mixed with 10 mL of scintillation cocktail (Ultima Gold, Boston, MA) and analyzed using a liquid scintillation counter (Beckman Coulter LS 6500 Multipurpose Scintillation Counter, Fullerton, CA). In order to ensure that the integrity of HEM was not compromised in the transport experiment, HEM electrical resistance was determined again after the experiment using the same method stated earlier. To determine the effect of the one minute ethanol treatment on the permeation of CS across HEM, PBS instead of ethanol was used to treat the HEM samples and served as the PBS control. The steady-state permeability coefficient of HEM for CS was calculated by:
| (1) |
where CD is the donor chamber concentration of the model permeant (CS), A is the available diffusional area of the side-by-side diffusion cell, and dQ/dt is the slope of the linear region of the cumulative amount of the permeant in the receiver chamber against time plot. The permeability coefficient (P) can also be expressed as:
| (2) |
where D is the effective diffusion coefficient, K is the effective partition coefficient, and L is the effective transport path length across the membrane.
HEM Transport Experiments: Enhancer Deposition from Volatile Solvent (Enhancer/Ethanol Treatment)
HEM samples were allowed to thaw and placed on a PBS-wetted support as stated under “HEM Transport Experiments: Control.” The samples were then treated with 0.35 mL of 8% v/v enhancer (IPM, OA, UD, DoP, OS, AZ, or PADO) in ethanol. The 8% enhancers were believed to be sufficient to saturate the SC lipids, based on the volume of intercellular lipid in HEM SC used in the present study (~ 20% of the SC volume); the amounts of enhancers used were greater than the total mass of HEM SC lipid domain. After enhancer/ethanol treatment (the treatment of enhancer in ethanol), HEM samples were allowed to equilibrate on the PBS-wetted support for 20 minutes. Then, HEM samples were rinsed three times with PBS and patted gently with Kimwipes between rinses. After rinsing, HEM was mounted in a side-by-side diffusion cell and allowed to equilibrate for 2 hours with stirring at 37° C. This second equilibration step allowed the redistribution of enhancer within the SC [2]. Both diffusion cell chambers were then completely replaced with fresh PBS, and the transport experiment was carried out as described in “HEM Transport Experiments: Control.”
Calculation of Enhancement Factor on CS Permeation
The enhancement factor (E) of CS transport across the lipoidal pathway was determined by the ratio of the permeability coefficients of the permeant of enhancer/ethanol treated HEM to that of ethanol-treated HEM control:
| (3) |
where PL,enhancer is the permeability coefficient of HEM lipoidal pathway after enhancer/ethanol treatment, and PL,control is the permeability coefficient of the lipoidal pathway of ethanol-treated HEM (control) from the same skin donor. Since the enhancers used in the transport experiments have log Koct ≥4.2 and have low aqueous solubility, a correction for CS thermodynamic activity due to the presence of enhancer in the vehicle was not necessary [2].
Enhancer Depletion and HEM Barrier Recovery
To examine the duration for which an enhancer can sustain its enhancement effect in a volatile solvent system and whether the duration is related to enhancer depletion from HEM and hence the lipophilicity of an enhancer, HEM barrier recovery after PHE, OC, and OP (enhancers of log Koct ≤ 3.3) treatment was investigated. In practice, it was also important to determine the ability of HEM to recover from treatment of these topical volatile systems. In this study, HEM samples were treated with 8% v/v PHE, OC, or OP in ethanol and passive transport experiments were conducted as stated in “HEM Transport Experiments: Enhancer Deposition from Volatile Solvent (Enhancer/Ethanol Treatment).”
E2β Uptake and Partitioning into Human Stratum Corneum: Human Stratum Corneum Preparation
The SC was isolated from HEM using a method modified from previous protocols [24, 25]. Briefly, HEM was gently placed, with viable epidermis facing down in a Petri dish containing 0.2% trypsin in 0.15 M PBS (pH = 7.4). The Petri dish was then covered and kept at 37° C for 16 hours allowing digestion of the viable epidermis. After digestion, the SC floating on the surface was lifted up using a filter paper. The SC was allowed to float over de-ionized water where it was freed from the filter paper after which the SC was lifted and the viable epidermis side was wiped gently using a cotton swab. Then, the SC was rinsed at least three times using distilled de-ionized water. After rinsing, the isolated SC was freed from excess water using Kimwipes. The SC samples were carefully weighed, placed in scintillation vials and then placed in a desiccator at room temperature for 12 hours. The samples were again weighed after drying and stored in a freezer until use.
The SC surface lipids were removed following the treatment established previously [26]. Briefly, dry SC was rinsed with n-hexane for 3 X 10 seconds and patted dry between each rinse using Kimwipes. After treatment, n-hexane treated SC was placed in scintillation vials and allowed to dry in a desiccator overnight at room temperature. N-hexane treated SC samples were then carefully weighed.
Delipidized SC samples were prepared by placing dry SC of known weight in scintillation vials filled with 10 mL chloroform/methanol (2:1) [25] and kept on a low speed shaker for 48 hours at room temperature. The SC was then removed and rinsed three times with fresh chloroform/methanol (2:1). Delipidized SC was then placed in scintillation vials and allowed to dry in a desiccator at room temperature.
E2β Uptake and Partitioning with n-Hexane Treated and Delipidized SC
N-hexane treated (or delipidized) SC was placed in scintillation vials containing 10 mL of PBS for at least 4 hours to allow complete hydration. Fully hydrated SC (n-hexane treated or delipidized) was then carefully weighed to determine its water content. After hydration, SC (n-hexane treated or delipidized) was placed in a Petri dish containing PBS and allowed to float on the PBS. The SC (n-hexane treated or delipidized) was then lifted up with a filter paper, and the SC-filter composite was placed on a cotton support wetted with PBS. SC enhancer treatment was performed as described in “HEM Transport Experiments: Enhancer Deposition from Volatile Solvent (Enhancer/Ethanol Treatment)” with 0.35 mL of 8% v/v enhancer in ethanol. The SC (n-hexane treated or delipidized) was then allowed to equilibrate for 20 minutes. After equilibration, the SC (n-hexane treated or delipidized) was rinsed with PBS at least three times and patted dry using Kimwipes after each rinse. Then, the SC (n-hexane treated or delipidized) was carefully placed in scintillation vials containing 20 mL of E2β suspension (15 mg in 10 mL of PBS). The scintillation vials were sealed with parafilm and placed in a shaker at 37° C for 12 hours [25]. After the 12 hours of equilibration, the SC (n-hexane treated or delipidized) was carefully removed using tweezers and then rinsed with de-ionized water three times and patted dry with Kimwipes between rinses. Then, the SC (n-hexane treated or delipidized) was placed in scintillation vials, where 5 mL of absolute HPLC grade ethanol was added. E2β was extracted into ethanol in the vials for 48 hours at room temperature on a low speed shaker. The ethanol solutions were then conveyed into Pyrex culture tubes, centrifuged for 30 min at 3400 rpm (Fisher Centrific Model 228). The supernatant was filtered (first portion of filtrate was discarded) through a 0.45-μm Millipore filter (MF™ membrane, Bioscience, life Science Products) and was analyzed to determine E2β uptake into n-hexane treated and delipidized SC. N-hexane treated or delipidized SC samples following the same protocol treated with 0.35 mL ethanol without enhancer were used as the control.
Calculation of E2β Uptake
The equilibrium uptake amount of E2β was calculatedby:
| (4) |
where E2βcorrected is the amount of E2β uptake into the n-hexane treated SC (or delipidized SC) expressed here in micromoles of E2β uptake per milligrams of dry n-hexane treated SC (or delipidized SC), E2βextracted is the amount of E2β extracted from the n-hexane treated SC (or delipidized SC), Wdry is the dry n-hexane treated SC weight (or dry delipidized SC weight), Wwet is the wet weight of n-hexane treated SC (or the wet delipidized SC weight), and Saq is the aqueous solubility of E2β. The second term in the equation was used to correct for the E2β in the aqueous compartment within the SC.
HPLC and GC Analysis
For enhancer analysis, the same HPLC and GC methods as described in a previous study were used [2]. For E2β analysis, the same HPLC system [2] was used with 50% v/v acetonitrile in water as the mobile phase at a flow rate of 1 mL/min and UV detector wavelength of 214 nm. Calibration curve was prepared in the mobile phase based on peak area measurements.
RESULTS
HEM Transport Experiments
The permeability coefficient of CS across HEM in the PBS control experiments in Table 1 is consistent with those shown in previous studies [2, 25, 27]. The permeability coefficient of ethanol-treated HEM for CS (the ethanol control) is also shown in Table 1. The one minute ethanol treatment did not affect the permeation of CS across HEM when compared to the PBS control (p = 0.22, t-test).
Table 1.
Permeability Coefficient and Lag Time of Corticosterone Transport across HEM and Permeation Enhancement Factor.
| HEM Treatment | Permeability coefficient of HEM for CS (10−7 cm/s)a | Lag Time in Minutes | Enhancement Factorb |
|---|---|---|---|
| PBS | 2.1 ± 0.7 | 47 ± 15 | NA |
| Ethanol (EtOH) | 1.7 ± 0.5 | 56 ± 4 | NA |
| DoP/EtOH | 33 ± 11 | 48 ± 16 | 23 ± 5 |
| OS/EtOH | 6.5 ± 5.7 | 47 ± 14 | 2.5 ± 0.9 |
| IPM/EtOH | 4 ± 1 | 46 ± 14 | 3 ± 1 |
| AZ/EtOH | 11 ± 2 | 58 ± 17 | 8 ± 1 |
| PADO/EtOH | 4 ± 2 | 39 ± 11 | 3 ± 2 |
| UD/EtOH | 19 ± 3 | 58 ± 12 | 14 ± 4 |
| OA/EtOH | 10 ± 4 | 49 ± 6 | 7 ± 2 |
| PHE/EtOHd | 2.2 ± 0.6 | NA | 1.5 ± 0.3c |
| OP/EtOHd | 1.7 ± 0.1 | NA | 1.2 ± 0.2c |
| OC/EtOHd | 1.4 ± 0.3 | NA | 1.0 ± 0.3c |
Mean ± SD (n ≥ 3).
Calculated using equation (3).
Enhancement factor not significantly different from unity, possibly due to enhancer depletion and the relatively low enhancer Koct.
Enhancers examined in the HEM barrier recovery study
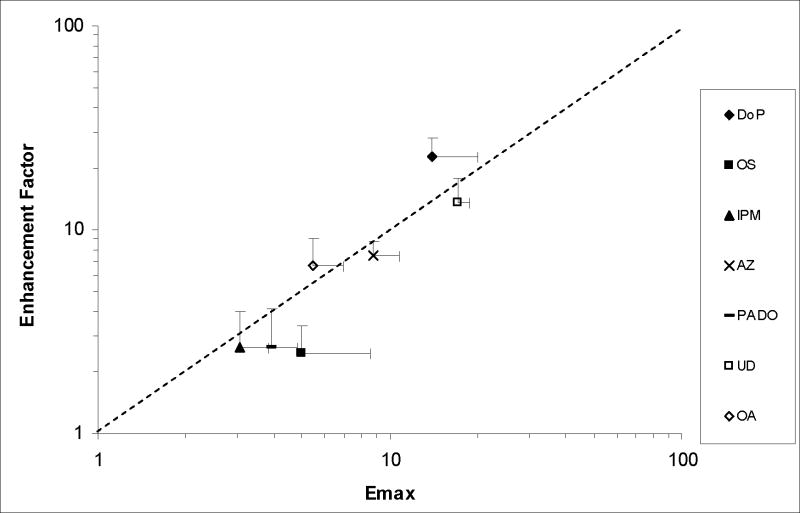
Table 1 column 2 presents the permeability coefficients of HEM for CS after enhancer/ethanol treatment. These permeability coefficients were used in the calculation of the enhancement factors, and the results are presented in Table 1 column 4. A comparison of these enhancement factors and Emax of the enhancers determined previously indicates a relationship between these two enhancement factors. Fig. 1 shows a correlation between the present enhancement factor and Emax (r2 = 0.72) with a slope of 1.17. The Emax values given in Fig. 1 was obtained previously [2]. The general correlation in the figure suggests similar permeation enhancement mechanisms induced by the enhancers following the treatment protocol in the present study and previous Emax studies.
Figure 1.
Enhancement Factor determined after Enhancer Deposition on HEM with the Volatile Solvent versus Emax of the Enhancer (n ≥ 3). Emax data were obtained from a previous study [2]. OP, PHE and OC (due to continuous enhancer depletion from HEM) enhancement factor were not included in this figure because the concentration of these enhancers within HEM did not remain constant throughout the transport experiment. The line represents a slope = 1.
The enhancers studied so far had low aqueous solubility and their partitioning from HEM into the equilibrating PBS in the donor and receiver chambers was assumed to be minimal. To validate this assumption, samples from the equilibrating PBS solution were analyzed for the enhancers and the concentration of the enhancers was found to be below detection limit using HPLC and GC (< 0.1–0.85 μg/mL). The absence of enhancers in the transport vehicle eliminates the necessity to correct for thermodynamic activity change of the permeant, hence the absence of a solubility ratio term in equation 3 [2].
Table 1 column 3 also presents the transport lag times of CS. The lag times of CS permeation across HEM were determined by extrapolating the linear regressions in the CS cumulative amount versus time plots to the abscissa. In all the transport experiments, steady-state transport was observed and the extrapolation showed linear regressions with r2 ≥ 0.98. The transport lag time data in the present study show that the lag time of CS permeation across HEM was relatively independent of the enhancement factor.
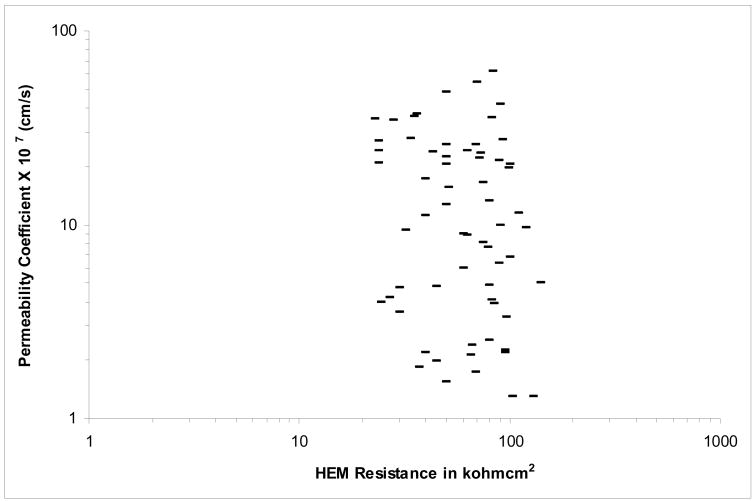
Electrical Resistance of HEM and Flux Enhancement across the Lipoidal Pathway
In the present study, the electrical resistance of HEM was used to probe the polar pathway across HEM [28]. Fig. 2 presents the HEM permeability coefficient of CS and HEM electrical resistance. The relatively high electrical resistance indicates that the polar pathway was not compromised by enhancer/ethanol treatment, and the contribution of the polar pathway to CS transport was negligible under the conditions in the present study. The data also show that the integrity of HEM remained essentially the same and above 15 kΩcm2 through the transport experiments.
Figure 2.
CS Permeability Coefficient versus HEM Electrical Resistance in the Transport Experiments of PBS control, Ethanol-Treated HEM Control, and Enhancer/Ethanol Treated HEM.
HEM Barrier Recovery and Enhancer Depletion
Table 1 column 2 also shows the permeability coefficients of HEM after treatment with the enhancers of relatively low lipophilicity (PHE, OP, and OC; log Koct ≤ 3.3) in the present study. Similar to the more lipophilic enhancers, the enhancement factor (Table 1, column 4) was determined by the ratio of the steady-state permeability coefficient of HEM after enhancer/ethanol treatment to that of the ethanol control. The enhancement factors of PHE, OP, and OC range from 0.7–1.8 (average: 1.0–1.5). Although these enhancers were found to provide a relatively high degree of permeation enhancement (based on their Emax values), their enhancement factors were not statistically different from unity, possibly due to the reversible enhancer effects and the depletion of the enhancers from HEM into the equilibrating PBS solution and transport vehicle [2].
E2β Uptake and Partitioning into SC
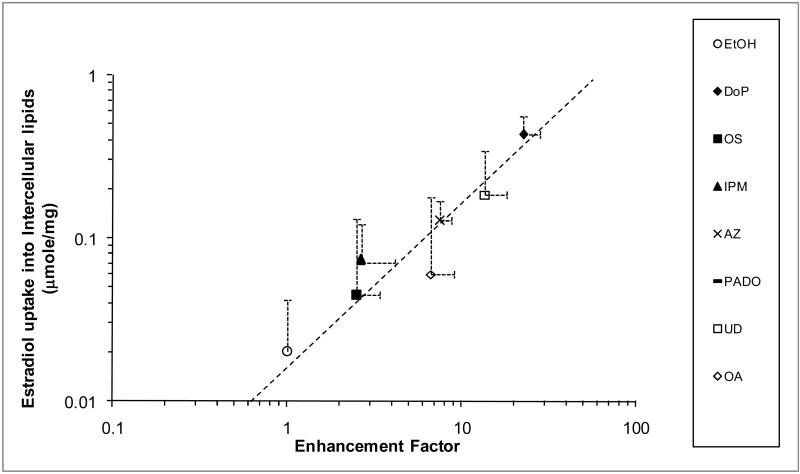
To understand the impact of the enhancers on permeant partitioning into the SC and SC lipid domain, the uptake of a lipophilic permeant E2β into the n-hexane treated SC and delipidized SC with and without enhancer/ethanol treatment was determined. Table 2 presents the amount of E2β uptake into the n-hexane treated SC and into the delipidized SC. The amount of E2β uptake into the SC intercellular lipid domain was calculated by subtracting the amount uptake into the delipidized SC from that into n-hexane treated SC [(Table 2 column 2)-(Table 2 column 4)]. Fig. 3 presents the plot of E2β uptake in the SC intercellular lipid domain and the enhancement factor determined in the permeation study with the same enhancer/ethanol treatment. A linear correlation was observed with a regression r2 = 0.92.
Table 2.
Estradiol Uptake in n-hexane Treated and Delipidized Human SC Treated with Enhancer/Ethanol.
| SC Treatment | Amount of E2β Uptake into n-Hexane Treated Human SCa | Amount of E2β Uptake into Delipidized Human SCa | |
|---|---|---|---|
| E2βcorrected,ib (μmole/mg Dry n-Hexane Treated Human SC) |
E2βcorrected,Ib (μmole/mg Dry Delipidized Human SC) |
E2βcorrected,ic (μmole/mg Dry Delipidized Human SC) |
|
| Ethanol (EtOH) | 0.03 ± 0.02 | 0.012 ± 0.005 | 0.011 ± 0.005 |
| DoP/EtOH | 0.5 ± 0.1 | 0.10 ± 0.03 | 0.08 ± 0.03 |
| OS/EtOH | 0.09 ± 0.08 | 0.05 ± 0.02 | 0.04 ± 0.02 |
| IPM/EtOH | 0.09 ± 0.04 | 0.02 ± 0.02 | 0.02 ± 0.02 |
| AZ/EtOH | 0.16 ± 0.04 | 0.039 ± 0.008 | 0.032 ± 0.007 |
| PADO/EtOH | 0.11 ± 0.05 | 0.05 ± 0.01 | 0.04 ± 0.01 |
| UD/EtOH | 0.3 ± 0.2 | 0.17 ± 0.08 | 0.14 ± 0.06 |
| OA/EtOH | 0.17 ± 0.09 | 0.13 ± 0.08 | 0.11 ± 0.07 |
| PHE/EtOHd | 0.08 ± 0.02 | 0.11 ± 0.06 | 0.09 ± 0.05 |
| OP/EtOHd | 0.09 ± 0.05 | 0.038 ± 0.007 | 0.032 ± 0.006 |
| OC/EtOHd | 0.028 ± 0.001 | 0.19 ± 0.06 | 0.16 ± 0.05 |
Mean ± SD (n ≥ 3).
Corrected for the uptake into the aqueous compartment.
Normalized by the weight of n-hexane treated SC. Hence the uptake data were multiplied by the weight percent of the delipidized component of SC (83.6%).
Enhancers examined in the HEM barrier recovery study
Figure 3.
Amount of E2β Uptake in the Intercellular Lipid Domain of the SC (expressed in micromoles of E2β in intercellular lipids per mg of dry SC) versus Enhancement Factor of an Enhancer (n ≥ 3). The line represents a slope = 1 in the log-log plot. The PADO data point and error bars overlap with those of IPM.
HEM Barrier Recovery and Effects on E2β Uptake
PHE, OP, and OC were studied to investigate the effects of enhancer/ethanol treatment of the less lipophilic enhancers (Koct ≤3.3) upon E2β partitioning. As shown in Table 2, PHE and OP enhancer treatment resulted in an increase in E2β uptake in both n-hexane treated and delipidized SC relative to the control, but there was no enhancement of E2β uptake into the SC lipid domain in the PHE case. The higher E2β uptake in n-hexane treated SC might be due to enhancer influence on the non-lipid domain evident by the essentially same E2β uptake in the n-hexane and delipidized SC and the lack of E2β uptake enhancement in the lipid domain. For OC, no enhancement of E2β partitioning into the n-hexane treated SC was observed. The higher E2β uptake into delipidized SC relative to the control could be due to enhancer induced E2β binding to the corneocytes after the removal of the SC lipid barrier. For PHE and OC, the negligible permeant partitioning enhancement in the lipid domain supports the hypothesis that the HEM barrier partially recovered after the treatment of these relatively low lipophilic enhancers, attributed to the depletion of the enhancer from the SC lipid domain in the uptake experiments. For OP, a relatively small increase in the amount of E2β uptake into the SC lipid domain was observed. The higher E2β uptake could be a result of partitioning enhancement due to the OP residue in the E2β equilibrating solution. The discrepancy between the uptake and permeation enhancement observed in the OP study (3x vs. 1.2x, respectively) could be related to the different procedures employed. In the transport study, the HEM was rinsed and equilibrated with PBS for 2 hours after the enhancer/ethanol treatment and then the equilibrating solution was completely replaced before the start of the permeation experiment whereas in the uptake study, the SC was placed in the E2β equilibrating solution after the enhancer/ethanol treatment and rapid rinsing.
DISCUSSION
Effect of Ethanol on HEM Permeation
Ethanol is commonly used in topical and transdermal products because it is a good solvent for many organic compounds thus facilitating the formulation of enhancer solutions at considerably high concentrations. Ethanol has high volatility. The surface tension of ethanol, being lower than the critical surface tension of skin [29], would result in ethanol spreading on HEM surface and allow uniform deposition of the enhancer. The impact of the short exposure of HEM to ethanol on CS permeation across HEM was determined in the present study. Despite ethanol being a well know enhancer, the continuous presence of ethanol is required to maintain its permeation enhancement effect on HEM [19], making this effect reversible. Evaporation of ethanol occurred within one minute after its application in the present study, and thus no effect of ethanol on the permeation of CS across HEM was found.
Effect of Ethanol on Enhancer Deposition and Enhancement Factor
The present data allow the investigation of the effect of ethanol (the volatile solvent) on enhancer deposition and possible synergistic effect between ethanol and the enhancer. It is hypothesized that the mechanism of enhancer presentation to SC using a volatile solvent system involves solvent evaporation leaving the enhancer in its pure state uniformly deposited on the HEM surface, subsequently enhancer partitioning into the skin reaching its maximum thermodynamic activity in the SC intercellular lipid domain similar to that of an infinite enhancer dose in a previous study [2], and finally the induction of permeation enhancement similar to Emax. It should be noted that the dose of enhancer on the surface of the skin was sufficient to saturate the SC intercellular lipid, and because the enhancers used in the present study posed low volatility, enhancer loss due to evaporation was minimal. This hypothesis is supported by the correlation between the enhancement factor in the present study and Emax (Fig. 1); there was no statistical difference between the enhancement factors in Table 1 compared to the Emax values of the enhancers in the previous study [2]. The results indicate no synergistic effect between ethanol and the studied enhancers on transdermal permeation enhancement. In addition, there was no enhanced enhancer presentation due to occurrence of enhancer in a kinetic supersaturated form on the HEM surface caused by rapid evaporation of the solvent system [30] under the experimental conditions employed in the present study. The correlation also suggests that the permeation enhancement mechanism of the enhancer via volatile solvent deposition using ethanol would be similar to that suggested in our previous study, and thus when the enhancer concentration in the SC increases, permeation enhancement increases [2].
E2β Uptake and Partitioning into SC
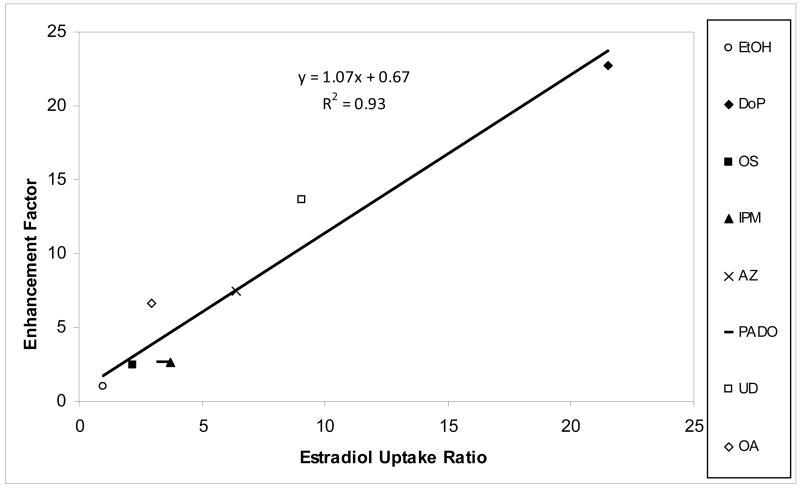
Two important insights were obtained in the present E2β uptake study. First, the enhancement of E2β uptake into SC demonstrates that the enhancers can improve drug loading into SC in the volatile solvent delivery system using ethanol. Drug loading enhancement is important in these systems due to the small finite doses applied to the skin and the potential of drug removal from the skin surface after application, e.g., via direct contact of clothing. Second, the E2β data provide insights into the mechanism of the enhancers. The direct correlation in Fig. 4 suggests that the transport rate determining pathway of CS in the SC, enhancer site of action, and intercellular lipid domain probed by E2β uptake in the present experiments are likely the same. With the vast chemical classes of the enhancers studied, this direct correlation also supports the hypothesis that the enhancer mechanism of action involves non-specific interactions between the enhancer and SC lipid domain in general. This will be further discussed in the next section.
Figure 4.
Relationship between CS Permeation Enhancement Factor and the Ratio of E2β Uptake in the Intercellular Lipid Domain of Enhancer/Ethanol Treated SC to Ethanol-Treated Control SC.
Equilibrium Partition Enhancement and Transport Rate Limiting Domain
According to equations 2 and 3 and assuming that the effective transport path length for lipophilic permeants in SC is relatively constant [31], the enhancement factor expression can be reduced to the ratio of the product of diffusion coefficient and partition coefficient of the permeant in enhancer/ethanol treated HEM to that of HEM control. The lag time shown in Table 1 column 3 (and consequently diffusion coefficient of permeant) suggest that the permeation enhancement mechanism of these enhancers is not related to the enhancement of CS diffusion within SC but the enhancement of CS partitioning into the SC intercellular lipids. This hypothesis is further supported by the linear regression slope of 1.07 (r2 = 0.93) in the permeation and uptake enhancement correlation (Fig. 4). The slope of unity suggests that the observed permeation enhancement is mainly attributed to the enhancement of CS partitioning into the SC. This finding is similar to that reported in the literature where drug partitioning into skin increased in the presence of an enhancer while diffusivity of permeant remained relatively constant [32]. The enhancement of E2β partitioning into the intercellular lipid domain could be due to an (a) increase in the solubility of E2β in the lipid domain related to changes in its polarity and/or (b) alteration of the intercellular lipid free volume. The increase in permeant solubility in the rate limiting domain would seem plausible since any changes in the free volume would consequently alter permeant diffusivity, which is not evident in the present study.
Enhancer Efficiency and Emax in Transdermal Products
A large number of topical products such as topical aerosols or sprays, hydro-alcoholic and polymer matrix gels utilize a solvent carrier system that evaporates after a transient period of time, leaving a drug and chemical enhancer on the skin surface [33, 34]. It has been hypothesized that enhancer deposition on the surface of skin would result in permeation enhancement that approaches Emax because the deposited enhancer would be in its neat form at its highest thermodynamic activity [2]. The results in Fig. 1 support this hypothesis. The correlation between the present enhancement factor and Emax indicates the possibility of using Emax to estimate enhancer effects in these systems for lipophilic enhancers (e.g., enhancers of Koct >3.3). As Emax can be predicted from enhancer hypothetical solubility in n-octanol (or the product of octanol-water partition coefficient and aqueous solubility “Koct x Sw”) [2], both permeation enhancement and skin drug uptake in these topical products can be theoretically calculated using these enhancer parameters. In addition, together with the previously demonstrated correlation between Emax and enhancer solubility in silicone elastomer [2], the permeation and partitioning enhancement correlations in the present study would imply that silicone elastomer can be a screening tool in topical and transdermal formulation testing. The ability to use a rapid screening tool for enhancer selection based on a simple solubility study would reduce the random in vitro enhancer screening using human skin that is both time consuming and with little known clear guidelines. For the relatively less lipophilic enhancers (e.g., enhancers PHE, OP, and OC, with Koct ≤3.3), the enhancers might deplete in the SC due to clearance over time and this would result in limited permeation enhancement as discussed in the previous study on Emax [2]. The direct correlation between E2β uptake ratio in enhancer treated SC and CS permeation enhancement further supports that Emax can be used as a general model to determine drug permeation enhancement induced by an enhancer given that the transport rate limiting domain is the lipoidal pathway and drugs of similar molecular sizes as CS are used [35]. It should be noted that a number of fatty acids have been studied in a similar manner in our laboratory. A more complex correlation between Emax and enhancer efficiency exists among the fatty acids due to the inclusion of fatty acids in both solid and liquid states in the testing, the effects of solvents upon the deposition and re-crystallization of the solid enhancers on the SC, and the influence of pH and enhancer pKa. These fatty acid results will be discussed in future work.
CONCLUSION
A number of transdermal and topical products containing steroids in volatile solvent systems (i.e. progesterone or testosterone) such as hydro-alcoholic gels as well as spray systems are available in the market. Once applied, the solvent in these products evaporates depositing the drug and the chemical enhancer on the skin for drug absorption. In order to select a proper enhancer in these products, the mechanism of the enhancer and the interplay between the enhancer and solvent should be understood. In the present study, a general correlation was found between the permeation enhancement due to the deposition of enhancer from the volatile solvent ethanol and Emax. This finding suggests that the enhancement is attributed solely to the enhancer and there is practically no effect (synergistic effect) of the volatile solvent system upon permeation enhancement. The enhancement factor vs. Emax correlation also suggests that enhancer presentation into skin from these topical and transdermal systems is likely via (a) solvent evaporation leaving the enhancer in its pure state in equilibrium with the SC and (b) enhancer partitioning in the skin approaching its maximum thermodynamic activity similar to its pure state and inducing permeation enhancement similar to Emax. The correlation also suggests that the permeation enhancement mechanism in these systems is consistent with those proposed in our previous study. The general permeation enhancement effects induced by the enhancers in these topical and transdermal systems can therefore be estimated using the enhancer “Koct x Sw” parameter or silicone polymer solubility as previously described. The present reversibility study suggests that the higher lipophilic enhancers (Koct >3.3) would sustain their enhancement effect for a longer duration and therefore would be more effective than the less lipophilic enhancers. In addition to the mechanism of enhancer presentation and examining the predictivity of Emax, the present study also provided insights into the effects of the enhancers upon drug loading into SC and the mechanism of action of these enhancers. In the skin uptake study, the enhancement of permeant uptake into the SC intercellular lipid domain was shown to correlate with the enhancement of permeant transport across HEM. This correlation suggests that the mechanism of enhancement of the enhancers is related the enhancement of permeant partitioning into the SC lipid domain. Thus, enhanced drug loading in the skin can also be achieved by the enhancers.
Acknowledgments
This research was supported by NIH Grant GM 063559. The authors thank Dr. Jinsong Hao and Dr. Qingfang Xu for their help in the laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kanikkannan N, Kandimalla K, Lamba SS, Singh M. Structure-activity relationship of chemical penetration enhancers in transdermal drug delivery. Current Medicinal Chemistry. 2000;7(6):593–608. doi: 10.2174/0929867003374840. [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim SA, Li SK. Effects of Chemical Enhancers on Human Epidermal Membrane: Structure-Enhancement Relationship based on Maximum Enhancement (Emax) Journal of Pharmaceutical Sciences. 2009;98(3):926–944. doi: 10.1002/jps.21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan TM, Reed BL, Finnin BC. Enhanced skin permeation of sex hormones with novel topical spray vehicles. Journal of Pharmaceutical Sciences. 1998;87(10):1213–1218. doi: 10.1021/js980025k. [DOI] [PubMed] [Google Scholar]
- 4.Morgan TM, Parr RA, Reed BL, Finnin BC. Enhanced transdermal delivery of sex hormones in swine with a novel topical aerosol. Journal of Pharmaceutical Sciences. 1998;87(10):1219–1225. doi: 10.1021/js980026c. [DOI] [PubMed] [Google Scholar]
- 5.El-Gibaly I, Mohamed FA, Shehata M. Effect of some penetration enhancers on release of clotrimazole from different gel formulations and histological changes of rabbit skin. Pharmazeutische Industrie. 1998;60(12):1088–1095. [Google Scholar]
- 6.Shin SC, Cho CW, Oh IJ. Effects of non-ionic surfactants as permeation enhancers towards piroxicam from the poloxamer gel through rat skins. International Journal of Pharmaceutics. 2001;222(2):199–203. doi: 10.1016/s0378-5173(01)00699-8. [DOI] [PubMed] [Google Scholar]
- 7.Rhee YS, Huh JY, Park CW, Nam TY, Yoon KR, Chi SC, Park ES. Effects of vehicles and enhancers on transdermal delivery of clebopride. Archives of Pharmacal Research. 2007;30(9):1155–1161. doi: 10.1007/BF02980252. [DOI] [PubMed] [Google Scholar]
- 8.Chik Z, Johnston A, Tucker AT, Chew SL, Michaels L, Alam CAS. Pharmacokinetics of a new testosterone transdermal delivery system, TDS (R)-testosterone in healthy males. British Journal of Clinical Pharmacology. 2006;61(3):275–279. doi: 10.1111/j.1365-2125.2005.02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith EW, Maibach HI. Percutaneous Penetration Enhancers. CRC Press; Boca Raton, FL: 2005. [Google Scholar]
- 10.Morgan TM, O’Sullivan HMM, Reed BL, Finnin BC. Transdermal delivery of estradiol in postmenopausal women with a novel topical aerosol. Journal of Pharmaceutical Sciences. 1998;87(10):1226–1228. doi: 10.1021/js9800275. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto H, Hashida M, Sezaki H. Effect of 1-Alkyl- or 1-Alkenylazacycloalkanone Derivatives on the Penetration of Drugs with Different Lipophilicities through Guinea-Pig Skin. Journal of Pharmaceutical Sciences. 1991;80(1):39–45. doi: 10.1002/jps.2600800111. [DOI] [PubMed] [Google Scholar]
- 12.Hadgraft J, Peck J, Williams DG, Pugh WJ, Allan G. Mechanisms of action of skin penetration enhancers retarders: Azone and analogues. International Journal of Pharmaceutics. 1996;141(1–2):17–25. [Google Scholar]
- 13.Kim YC, Park JH, Ludovice PJ, Prausnitz MR. Synergistic enhancement of skin permeability by N-lauroylsarcosine and ethanol. International Journal of Pharmaceutics. 2008;352(1–2):129–138. doi: 10.1016/j.ijpharm.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Warner KS, Shaker DS, Molokhia S, Xu QF, Hao JS, Higuchi WI, Li SK. Silicone elastomer uptake method for determination of free 1-alky-2-pyrrolidone concentration in micelle and hydroxypropyl-beta-cyclodextrin systems used in skin transport studies. Journal of Pharmaceutical Sciences. 2008;97(1):368–380. doi: 10.1002/jps.21094. [DOI] [PubMed] [Google Scholar]
- 15.Ghafourian T, Zandasrar P, Hamishekar H, Nokhodchi A. The effect of penetration enhancers on drug delivery through skin: a QSAR study. Journal of Controlled Release. 2004;99(1):113–125. doi: 10.1016/j.jconrel.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 16.He N, Warner KS, Chantasart D, Shaker DS, Higuchi WI, Li SK. Mechanistic study of chemical skin permeation enhancers with different polar and lipophilic functional groups. Journal of Pharmaceutical Sciences. 2004;93(6):1415–1430. doi: 10.1002/jps.20030. [DOI] [PubMed] [Google Scholar]
- 17.Warner KS, Li SK, He N, Suhonen TM, Chantasart D, Bolikal D, Higuchi WI. Structure-activity relationship for chemical skin permeation enhancers: Probing the chemical microenvironment of the site of action. Journal of Pharmaceutical Sciences. 2003;92(6):1305–1322. doi: 10.1002/jps.10367. [DOI] [PubMed] [Google Scholar]
- 18.Kang L, Yap CW, Lim PFC, Chen YZ, Ho PC, Chan YW, Wong GP, Chan SY. Formulation development of transdermal dosage forms: Quantitative structure-activity relationship model for predicting activities of terpenes that enhance drug penetration through human skin. Journal of Controlled Release. 2007;120(3):211–219. doi: 10.1016/j.jconrel.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Kim YH, Ghanem AH, Mahmoud H, Higuchi WI. Short Chain Alkanols as Transport Enhancers for Lipophilic and Polar Ionic Permeants in Hairless Mouse Skin -Mechanism(s) of Action. International Journal of Pharmaceutics. 1992;80(1):17–31. [Google Scholar]
- 20.Yoneto K, Ghanem AH, Higuchi WI, Peck KD, Li SK. Mechanistic Studies of the 1-Alkyl-2-Pyrrolidones as Skin Permeation Enhancers. Journal of Pharmaceutical Sciences. 1995;84(3):312–317. doi: 10.1002/jps.2600840310. [DOI] [PubMed] [Google Scholar]
- 21.He N, Li SK, Suhonen TM, Warner KS, Higuchi WI. Mechanistic study of alkyl azacycloheptanones as skin permeation enhancers by permeation and partition experiments with hairless mouse skin. Journal of Pharmaceutical Sciences. 2003;92(2):297–310. doi: 10.1002/jps.10269. [DOI] [PubMed] [Google Scholar]
- 22.Peck KD, Ghanem AH, Higuchi WI. The Effect of Temperature Upon the Permeation of Polar and Ionic Solutes through Human Epidermal Membrane. Journal of Pharmaceutical Sciences. 1995;84(8):975–982. doi: 10.1002/jps.2600840813. [DOI] [PubMed] [Google Scholar]
- 23.Beverley KJ, Clint JH, Fletcher PDI. Evaporation rates of pure liquids measured using a gravimetric technique. Physical Chemistry Chemical Physics. 1999;1(1):149–153. [Google Scholar]
- 24.Raykar PV, Fung MC, Anderson BD. The Role of Protein and Lipid Domains in the Uptake of Solutes by Human Stratum-Corneum. Pharmaceutical Research. 1988;5(3):140–150. doi: 10.1023/a:1015956705293. [DOI] [PubMed] [Google Scholar]
- 25.Chantasart D, Sa-Nguandeekul P, Prakongpan S, Li SK, Higuchi WI. Comparison of the effects of chemical permeation enhancers on the lipoidal pathways of human epidermal membrane and hairless mouse skin and the mechanism of enhancer action. Journal of Pharmaceutical Sciences. 2007;96(9):2310–2326. doi: 10.1002/jps.20865. [DOI] [PubMed] [Google Scholar]
- 26.Chantasart D, Li SK, He N, Warner KS, Prakongpan S, Higuchi WI. Mechanistic studies of branched-chain alkanols as skin permeation enhancers. Journal of Pharmaceutical Sciences. 2004;93(3):762–779. doi: 10.1002/jps.10550. [DOI] [PubMed] [Google Scholar]
- 27.Al-Qallaf B, Das DB, Mori D, Cui Z. Modelling transdermal delivery of high molecular weight drugs from microneedle systems. Philosophical Transactions of the Royal Society a-Mathematical Physical and Engineering Sciences. 2007;365(1861):2951–2967. doi: 10.1098/rsta.2007.0003. [DOI] [PubMed] [Google Scholar]
- 28.Peck KD, Higuchi WI. Characterization of the Passive Transdermal Diffusional Route of Polar/Ionic Permeants. In: Potts RO, Guy RH, editors. Mechanisms of Transdermal Drug Delivery. Marcel Dekker, Inc.; New York, New York: 1997. pp. 267–290. [Google Scholar]
- 29.Elkhyat A, Agache P, Zahouani H, Humbert P. A new method to measure in vivo human skin hydrophobia. Int J Cosmet Sci. 2001;23(6):347–352. doi: 10.1046/j.0412-5463.2001.00108.x. [DOI] [PubMed] [Google Scholar]
- 30.Reid ML, Brown MB, Jones SA. Manipulation of Corticosteroid Release from a Transiently Supersaturated Topical Metered Dose Aerosol Using A Residual Miscible Co-Solvent. Pharm Res. 2008;25(11):2573–2580. doi: 10.1007/s11095-008-9675-3. [DOI] [PubMed] [Google Scholar]
- 31.Yu B, Dong CY, So PTC, Blankschtein D, Langer R. In vitro visualization and quantification of oleic acid induced changes in transdermal transport using two-photon fluorescence microscopy. Journal of Investigative Dermatology. 2001;117(1):16–25. doi: 10.1046/j.0022-202x.2001.01353.x. [DOI] [PubMed] [Google Scholar]
- 32.Yamashita F, Yoshioka T, Koyama Y, Okamoto H, Sezaki H, Hashida M. Analysis of Skin Penetration Enhancement Based on a 2-Layer Skin Diffusion-Model with Polar and Nonpolar Routes in the Stratum-Corneum - Dose-Dependent Effect of 1-Geranylazacycloheptan-2-One on Drugs with Different Lipophilicities. Biological & Pharmaceutical Bulletin. 1993;16(7):690–697. doi: 10.1248/bpb.16.690. [DOI] [PubMed] [Google Scholar]
- 33.Hadgraft JW. Recent progress in the formulation of vehicles for topical applications. Br J Dermatol. 1972;87(4):386–389. doi: 10.1111/j.1365-2133.1972.tb07428.x. [DOI] [PubMed] [Google Scholar]
- 34.Bronaugh RL, Maibach HI. Topical Absorption of Dermatological Products. Marcel Dekker; New York, NY: 2002. [Google Scholar]
- 35.Li SK, Higuchi WI. Mechanistic Studies of Permeation Enhancers. In: Smith EW, Maibach HI, editors. Percutaneous Penetration Enhancers. CRC Press, Inc.; Boca Raton, FL: 2005. pp. 271–292. [Google Scholar]