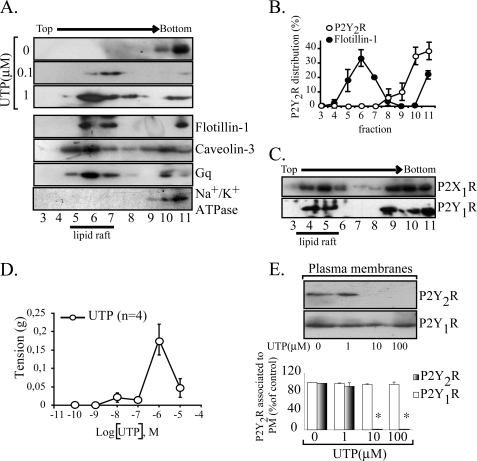
FIGURE 1.
UTP changes the plasma membrane distribution of the P2Y2R. The association of the P2Y2R with raft domains was assessed in sodium carbonate extracts separated in sucrose gradients; membrane rafts generally correspond to fractions 4–7 that were enriched with the endogenous markers flotillin-1 and caveolin-3. In non-stimulated HCA, the P2Y2R localized exclusively in fractions 9–11 co-migrating with the non-membrane raft protein Na+/K+ ATPase subunit β (representative immunoblots of three independent experiments are shown). Furthermore, the P2Y2R signaling partner Gq was distributed in raft and non-raft fractions (A). The P2Y2R associates with membrane rafts upon agonist stimulation. HCA segments were perfused ex vivo with different concentrations of UTP for 4 min (n = 3 each). Immunoblots from sucrose density gradients show that 0.1–1 μm UTP caused a rapid and nearly complete P2Y2R enrichment in membrane rafts (A). For comparative purposes, densitometry of the sodium carbonate immunoblots for P2Y2R and flotillin-1 are shown in B. Symbols, average values; bars, S.E. (n = 3). As a control, we observed that the P2Y1R and the P2X1R from these vessels partially localized in raft fractions (C). The contractile response elicited by UTP was plotted as a concentration-response curve; maximal response was attained with 1 μm, and larger concentrations elicited less response (D). Symbols, average values; bars, S.E. (n = 4). E, P2Y2R internalization induced at 10–100 μm UTP. HCA segments were perfused with 1, 10, or 100 μm UTP for 4 min; as controls, separated vessels were perfused with agonist-free buffer. Cells were harvested, SMC-plasma membranes were isolated by centrifugation, and 15 μg of these fractions were subjected to Western blot analysis using the indicated antibodies. Representative immunoblots are shown in the upper part of E; columns, mean values; bars, S.E. *, p < 0.05 as compared with the controls.