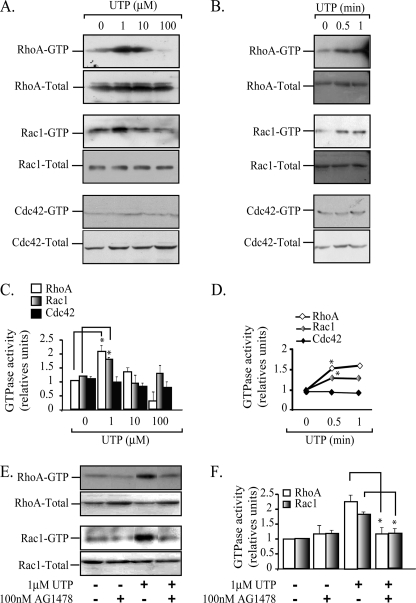
FIGURE 5.
The UTP-mediated activity of both RhoA and Rac1 is dependent on EGFR tyrosine kinase activity. The activation of both RhoA and Rac1, but not Cdc42, precedes the partition of the P2Y2R into membrane rafts. UTP-mediated activation of RhoA, Rac1, and Cdc42 was measured in HCA segments perfused with 1, 10, and 100 μm UTP; densitometric analysis showed a concentration-dependent activation of RhoA and Rac1, reaching a maximum at 1 μm UTP (A and C). Kinetic experiments using 1 μm UTP showed that the increase in the GTP loading of RhoA and Rac occurs within the first 30 s of UTP perfusion (B and D). Cdc42 was not activated under these conditions. Control experiments were performed in HCA segments perfused with agonist-free buffer. Data were normalized to the relative activation of the protein compared with the control and assigned an arbitrary value of 1; bars, S.E. (n = 3 per assay). *, p < 0.05 when comparing control versus UTP-perfused tissues. HCA segments were perfused sequentially with Krebs-Ringer buffer with or without 100 nm AG1478 for 30-min and the same buffer supplemented with 1 μm UTP for another 4 min. Activation of RhoA and Rac1 GTPases by UTP were completely blocked by the EGFR tyrosine kinase inhibitor (E and F). *, p < 0.05 as compared with the same protocol without AG 1478 (n = 3–4 per assay).