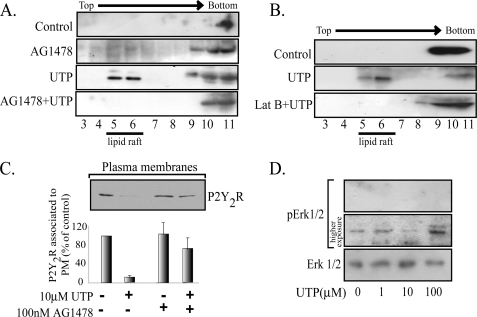
FIGURE 6.
The P2Y2R plasma membrane distribution is dependent on EGFR tyrosine kinase activity and actin cytoskeleton. The UTP-mediated partitioning of the P2Y2R into membrane rafts was also blocked by either AG1478 or 100 nm latrunculin B (A and B). HCA segments were perfused sequentially with Krebs-Ringer buffer with or without 100 nm AG1478 for 30 min and the same buffer supplemented with 10 μm UTP for another 4 min. As controls, separated vessels were perfused with agonist-free buffer. C shows that the P2Y2R internalization induced by 100 μm UTP is dependent on the EGFR tyrosine kinase activity. HCA segments were perfused with 100 μm UTP for 4 min; as controls, separated vessels were perfused with agonist-free buffer. Cells were harvested; SMC-plasma membranes were isolated by centrifugation; 15 μg of these fractions were subjected to Western blot analysis using the indicated antibody. Representative immunoblots are shown in the upper part of C. Columns, mean values; bars, S.E. HCA segments were perfused ex vivo with 0–100 μm UTP for 4 min. Cell-equivalent amounts of lysate were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membranes. Blots were probed with monoclonal anti-phospho-ERK antibodies and developed with the ECL enhanced chemiluminescence detection system (SuperSignalR West Femto, Pierce). As a loading control, membranes were stripped and probed for total ERK. A representative blot of three independent experiments is shown in D. No increases in the phospho-content of ERK were detected after UTP perfusions.