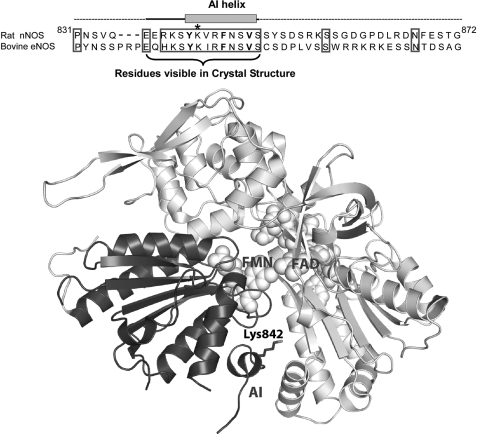
FIGURE 1.
Top, amino acid sequence of the AI in Rat nNOS and bovine eNOS. The residues comprising the AI helix and the portion of the AI that is visible in the nNOSred crystal structure are indicated. The three canonical hydrophobic residues in the putative CaM binding motif of the AI are in boldface type. Lys842 is marked with an asterisk. Bottom, ribbon diagram of the nNOSred crystal structure that highlights the relative position of the AI and Lys842 in the FMN subdomain. The FMN subdomain is colored black, and the rest of the reductase domain is gray. Only the AI residues that are visible in the crystal structure are shown. The bound FAD and FMN molecules are shown as space-filling models. This figure was drawn using Protein Data Bank file 1TLL.