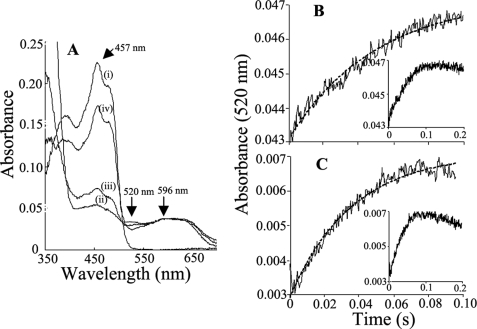
FIGURE 7.
Kinetics of electron transfer between FAD hydroquinone and FMN semiquinone in nNOSred and the K842E mutant. A, representative spectra recorded in air-saturated buffer at room temperature: oxidized WT nNOSred (i) and nNOSred immediately (ii) and 2 min (iii) after receiving a 10-fold molar excess of NADPH and after all of the NADPH had been oxidized (iv). Wavelengths that indicate general flavin reduction (457 nm), formation of the FAD semiquinone (520 nm), and general formation of flavin semiquinones (600 nm) are indicated. B and C, absorption change at 520 nm obtained after mixing the air-stable semiquinone forms of WT nNOSred (B) or K842E nNOSred (C) with NADPH. Final concentrations after mixing were as follows: nNOSred enzymes, 5 μm; NADPH, 50 μm. Lines of best fit are shown as dashed lines. Insets, absorbance changes recorded over a longer time period (200 ms). Kinetic traces shown are the average calculated from 8–10 mixing experiments each.