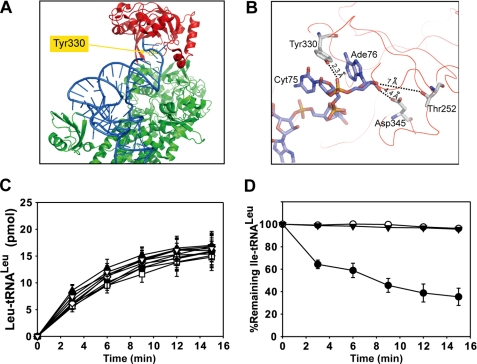
FIGURE 4.
Spatial location of mutated residues and effects of various mutants on aminoacylation and deacylation. A, structure of TtLeuRS in complex with tRNALeu (in blue) (2BYT). Residue numbering is from EcLeuRS. Tyr-330 is shown in yellow (spatial equivalent of AaY358). The editing domain (CP1) is colored in red, and the synthetic and C-terminal domain is in green. B, a view of the CP1 domain shows the last nucleotides from the tRNA and three crucial residues from the enzyme. Numbering is from EcLeuRS. Distances between nucleotides and residues are indicated. C, tRNALeu aminoacylation performed in the reaction mix containing 20 μm tRNALeu and 20 nm AaLeuRS (●) or mutated derivatives: ♦, AaLeuRS-Y358A; □, AaLeuRS-Y358L; △, AaLeuRS-Y358R; ■, AaLeuRS-Y358K; ○, AaLeuRS-Y358D; ▼, AaLeuRS-Y358E; ◇, AaLeuRS-Y358F; ▲, AaLeuRS-Y358W; ▽, AaLeuRS-Y358T. D, hydrolysis of 1 μm Ile-AatRNALeu by 20 nm AaLeuRS (●), AaLeuRS-Y358D (○), or AaLeuRS-Y358E (▼) is shown.