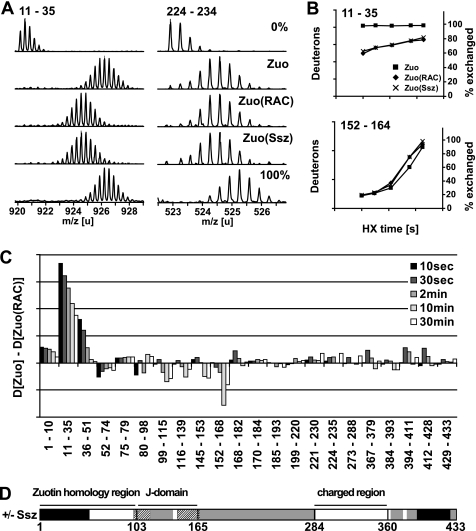
FIGURE 3.
Zuo N terminus is strongly stabilized by Ssz binding. A, HX properties of Zuo uncomplexed and bound to Ssz. Mass spectra of representative peptic peptides of Zuo (aa 11–35, m/z = 920,1776, z = +3; aa 224–235, m/z = 571,9333, z = +3) incubated in H2O (0%) or for 2 min in D2O in the uncomplexed state (Zuo), in the complexed state (Zuo(RAC)), and in the reconstituted complex (Zuo(Ssz)). 100% designates a control spectrum for the same peptides obtained from fully deuterated RAC. The samples during HX contained 0.5 μm Zuo, or 0.5 μm RAC, or 0.3 μm Zuo and 0.6 μm Ssz, and 0.6 mm ATP. B, kinetics of deuteron incorporation into selected segments of Zuo in the absence (■) and the presence of Ssz (Zuo(RAC), ♦; Zuo(Ssz), X). The axis on the left indicates the number of deuterons incorporated into the particular peptide, and the axis on the right the corresponding fraction exchanged in % of total exchangeable amides. C, difference of deuteron incorporation between Zuo and Zuo(RAC) after different incubation times (10 s to 30 min) in D2O. The data were resolved to individual peptic peptides as indicated by the start and end residue numbers of the corresponding segments (see also supplemental Fig. S3). D, schematic model of the domain structure of Zuo colored according to the observed HX protection patterns induced by Ssz binding. Black, HX protection upon Ssz binding; dashed, HX deprotection upon Ssz binding, gray, no changes in HX properties upon Ssz binding, and white, no data available. Protection and deprotection effects were considered that were ≥ 1 D and ≥ 10% of the total exchangeable sites for at least one HX time point measured in all peptides covering a particular region.