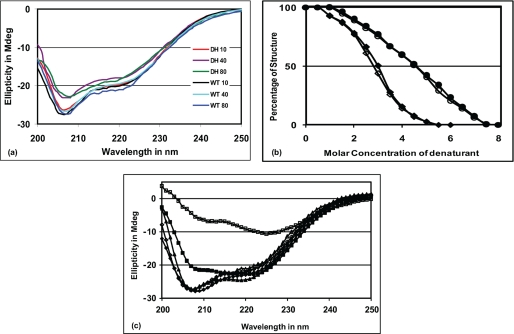
FIGURE 4.
CASQ2D307H mutant is relatively stable when treated with (a) temperature, (b) denaturants, but shows (c) altered behavior to calcium. Solid symbols are used for WT and open symbols are used for D307H protein. a, far-UV CD spectra of both proteins at 10, 40, and 80 °C shows that both proteins are equally stable at higher temperatures. b, unfolding curves of the two proteins in the presence of urea (●/○) and GdnHCl (♦/◇) are very similar. c, when [Ca2+] was increased from 0 to 5 mm, the CD spectrum of the mutant protein became drastically different from that of WT (■/□). Upon chelation of Ca2+ with EGTA (♦/◇), both proteins could regain CD ellipticity comparable with their native conformation at 25 °C (▴/Δ), although the WT protein needed only ∼3 mm EGTA, the mutant protein required ∼5.5 mm EGTA for the same.