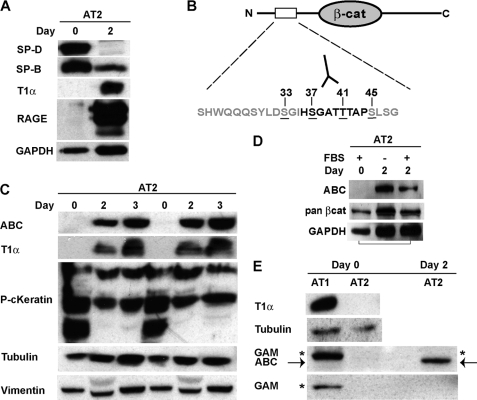
FIGURE 1.
Expression of the signaling form of β-catenin increases during trans-differentiation of AT2 cells in culture. AEC types 1 and 2 were isolated from rat lungs, and total protein was extracted immediately after isolation (Day0) or after 2–3 days of culture. Activated β-catenin (ABC), T1α and RAGE (AT1 markers), pancytokeratin (epithelial marker), SP-D and SP-B (AT2 markers), vimentin (fibroblast marker), and β-tubulin and GAPDH (loading controls) were detected by Western blot as described under “Materials and Methods.” A, AT2 cells express SP-D and SP-B on Day0, but begin to express T1α and RAGE by Day2. B, schematic of the N-terminal casein kinase 1α/GSK3β phosphorylation sites in β-catenin. ABC (mAb 8E7) specifically recognizes Ser-37 and Thr-41 in the unphosphorylated state (17). C, freshly isolated AT2 do not express ABC or T1α, but both proteins are up-regulated by Day2 and Day3 in culture. Platings from different cell isolates are shown in duplicate. D, detection of ABC in AT2 cells is not dependent on serum. E, freshly isolated AT1 express T1α, but not ABC. Goat anti-mouse secondary antibody recognizes a nonspecific band (asterisk) that closely co-migrates with ABC (arrow) in AT1, but not AT2 isolates. Because isolation of type 1 cells relies on positive selection with the T1α monoclonal antibody, we suspect the nonspecific band is due unreduced immunoglobulin.