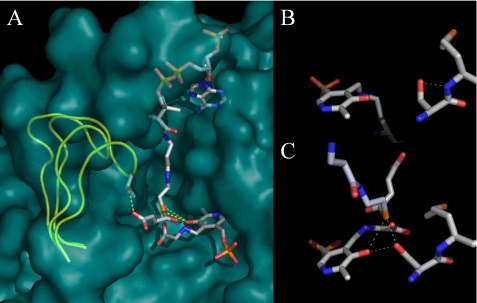
FIGURE 1.
Structural models for murine erythroid ALAS based on the R. capsulatus crystal structures. A, Michaelis complex modeled by alignment of open holoenzyme and closed glycine- and succinyl-CoA-bound monomeric structures. Serine 254 is hidden by the succinyl-CoA ester in this view from the perspective of the adjacent subunit, which has been removed. The active site loop is shown in a yellow cartoon for the open and closed conformations, and all other structural features are for the closed conformation. B, serine 254 in the open conformation. C, serine 254 in the closed conformation with succinyl-CoA bound.