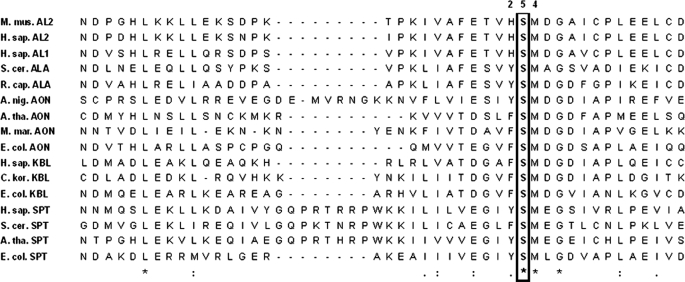
FIGURE 2.
Multiple sequence alignment of phylogenetically diverse members of the α-oxoamine synthase family in the region of murine ALAS2 serine 254. The amino acid sequences were retrieved from public data bases (NCBI) and aligned using ClustalW (42). The conserved serine residue is boxed and in boldface. The amino acid numbering (i.e. 254) refers to that of murine erythroid ALAS (mALAS2). Represented proteins are as follows: M. mus. AL2, Mus musculus erythroid ALAS (156255176); H. sap. AL2, Homo sapiens erythroid ALAS (28586); H. sap. AL1, H. sapiens housekeeping ALAS (40316939); S. cer. ALA, Saccharomyces cerevisiae ALAS (151942209); R. cap. ALA, R. capsulatus ALAS (974202); A. nig. AON, Aspergillus niger AONS (61696868); A. tha. AON, Arabidopsis thaliana AONS (42573269); M. mar. AON, Methanococcus maripaludis AONS (1599054); E. col. AON, E. coli AONS (85674759); H. sap. KBL, H. sapiens KBL (3342906); C. kor. KBL, Candidatus korarchaeum cryptofilum (17017433); E. col. KBL, E. coli KBL (169753078); H. sap. SPT, H. sapiens SPT (4758668); A. tha. SPT, A. thaliana SPT (17221603); S. cer. SPT, S. cerevisiae SPT (706828), E. col. SPT, E. coli SPT (170517920). AONS, 8-amino-7-oxononanoate synthase; SPT, serine palmitoyltransferase; KBL, 2-amino-3-oxobutyrate CoA ligase.