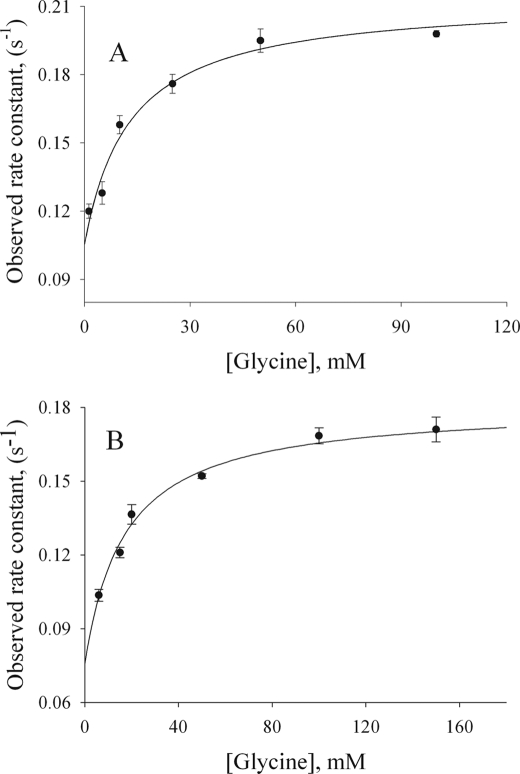
FIGURE 4.
Reaction of the Ser-254 variants (60 μm) with increasing concentrations of glycine. Data were fitted to Reaction 2 for a two-exponential process, yielding equilibrium and rate constants for S254T (KD = 1.5 ± 0.4 mm, k1 = 0.11 ± 0.01 s−1, and k−1 = 0.070 ± 0.004 s−1) (A) and S254A (KD = 6.6 ± 0.6 mm, k1 = 0.159 ± 0.04 s−1, and k−1 = 0.072 ± 0.001 s−1) (B). The fitted rate constants for wild-type ALAS yielded an overall binding KD of 8.0 ± 0.1 mm (18).