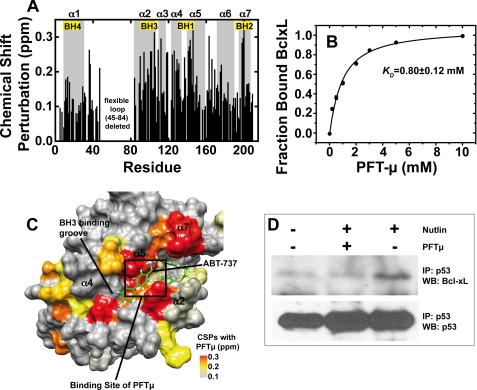
FIGURE 5.
Binding of PFTμ to BclxL and effect of PFTμ on the endogenous p53-BclxL complex. A, chemical shift perturbation data for the interaction between 300 μm 15N-BclxLΔLT and PFTμ probed with 15N-HSQC experiments in 10 mm sodium phosphate, pH 7.2, 1 mm DTT at 293 K. There are significant chemical shift changes at the BH123 binding pocket where BH3 peptides of pro-apoptotic BH3-only proteins bind. The BH regions are labeled with boxes. B, titration curve between 300 μm BclxLΔLT and PFTμ. The chemical shift changes upon subsequent titration of PFTμ were measured with 15N-HSQC spectra, normalized, and fitted with Equation 2 (see “Experimental Procedures”). A dissociation constant of 0.8 mm was obtained. Error bars obtained by evaluation of 5 individual residues in BclxL are smaller than the size of the symbols. C, chemical shift perturbations (CSPs) of BclxL in the presence of 10 mm PFTμ mapped onto the BclxL structure. For comparison, ABT-737, a previously published Bcl2/xL inhibitor, is shown in green. A box indicates the binding site for PFTμ obtained by NMR data driven docking (supplemental Fig. S8). D, effect of PFTμ on the endogenous BclxL-p53 interaction. RKO cells were incubated with 15 μm PFTμ and/or 10 μm Nutlin or left untreated. Endogenous p53 was immunoprecipitated with DO-1 antibody from equal amounts of cell lysates and the fraction of complexed BclxL was detected by Western blotting. IP, immunoprecipitation; WB, Western blot.