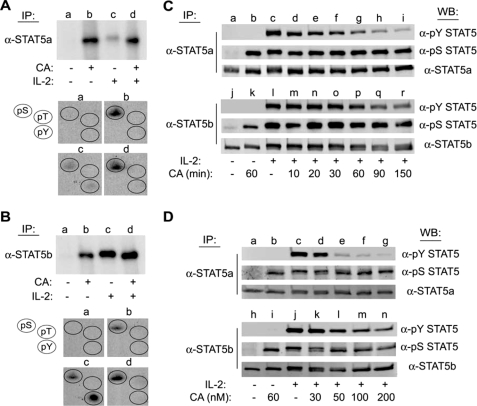
FIGURE 1.
CA induces serine phosphorylation and inhibits IL-2-mediated tyrosine phosphorylation of STAT5a/b in human lymphocytes. YT cells (1.5 × 107) were labeled with [32P]orthophosphate for 2 h at 37 °C and treated without (lanes a and c) or with 100 nm CA (lanes b and d) for 60 min prior to stimulation in the absence (lanes a and b) or presence of IL-2 (lanes c and d) for 10 min. Cell lysates were then immunoprecipitated (IP) with α-STAT5a (A) or α-STAT5b (B), separated by SDS-PAGE, and subjected to autoradiography (upper panels). The corresponding STAT5 bands were excised and subjected to phosphoamino acid analysis (groups a–d) (lower panels). The position of phosphoserine, -threonine, and -tyrosine standards (pS, pT, and pY) were detected by ninhydrin as indicated (circles). C, YT cells were left untreated (lanes a and j), treated with CA for 60 min (lanes b and k), stimulated with IL-2 for 10 min (lanes c and l), or pretreated with 100 nm CA for 10–150 min prior to stimulation with IL-2 for 10 min (lanes d–i and m–r). STAT5a (upper panel) and STAT5b (lower panel) were immunoprecipitated and Western blotted (WB) with the antibodies indicated. D, YT cells were left untreated (lanes a and h), treated with 100 nm CA for 90 min (lanes b and i), stimulated with IL-2 for 10 min (lanes c and j), or pretreated with 30–200 nm CA for 90 min followed by stimulation with IL-2 for 10 min (lanes d–g and k–n) as indicated. STAT5a (upper panel) and STAT5b (lower panel) proteins were immunoprecipitated and analyzed by Western blot using the antibodies indicated. Representative data from three independent experiments are shown.