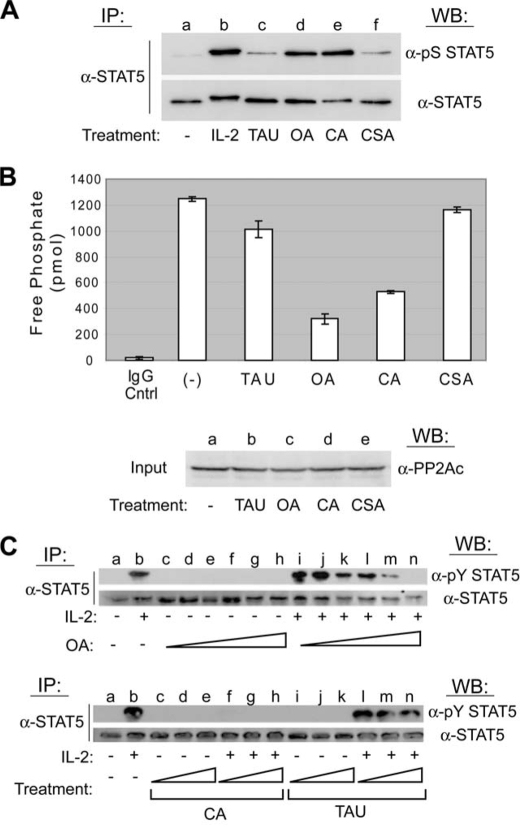
FIGURE 2.
PP2A, but not PP1 or PP2B, activity is required for IL-2-induced tyrosine phosphorylation of STAT5. A, YT cells were left untreated (lane a), stimulated with IL-2 for 30 min (lane b), or treated with inhibitory concentrations of the appropriate protein phosphatase uncoupler that included 1 μm TAU (lane c), 250 nm OA (lane d), 100 nm CA (lane e), or 250 nm CSA (lane f) for 60 min. STAT5 proteins were immunoprecipitated (IP), separated by SDS-PAGE, and analyzed by Western blot (WB) using the antibodies indicated. B, YT cells were left untreated or treated with 1 μm TAU, 250 nm OA, 100 nm CA, or 250 nm CSA for 60 min. PP2Ac proteins were immunoprecipitated and activity measured by serine/threonine phosphatase assays (upper panel). Normal mouse antiserum (IgG Cntrl) was used as a negative control for IP. The experiment was performed in triplicate where values are mean ± S.D. of free phosphate levels. Cell lysate was probed by Western blot using anti-PP2Ac to ensure equal protein input (lower panel). C, YT cells were left untreated (lanes a), stimulated with IL-2 for 30 min (lanes b), or treated with increasing concentrations of OA (10–200 nm) for 90 min followed by stimulation in the absence (lanes c–h, upper panel) or presence of IL-2 for 10 min (lanes i–n, upper panel). YT cells were similarly treated with CA (50–200 nm) or TAU (0.5–4 μm) for 90 min followed by stimulation in the absence (lanes c–e and i–k, respectively, lower panel) or presence of IL-2 for 30 min (lanes f–h and l–n, respectively, lower panel). STAT5 proteins were immunoprecipitated and analyzed by Western blot using the antibodies indicated. Representative data from three independent experiments are shown.